Abstract
Background: The Pipeline Vantage Flow diverter (Vantage) is the latest generation of Pipeline flow diverters introducing Cobalt-chronium drawn-filled tubing and 48 to 68 wires.
Purpose: We report the initial safety and efficacy of Vantage in treating intracranial aneurysms in the published literature.
Data Sources: A systematic review and meta-analysis was conducted according to established protocols. Searches were conducted in PubMed, Scopus, Web-of-Science, and Embase databases up to December 2023. Original studies reporting treatment outcomes for intracranial aneurysms using Vantage in more than five patients were included.
Study Selection: Pooled data from 5 studies (373 patients, 418 aneurysms) were analyzed.
Data Analysis: Outcomes of interest were: technical success, occlusion rates, complication outcomes and mortality.
Data Synthesis: A technical success rate of 99.2% (95% CI: 98.29%–100%) was found. In unruptured cases, success rate was 378/383 (99.6%) versus 17/20 (85.0%) in ruptured cases (P < .01). Complete occlusion rate was 74.3% (95% CI: 67.43%–80.59%), with no significant difference between ruptured and unruptured cases (P = 0.72); median of follow up 6 months. Overall mortality rate was 1.2% (95% CI: 0.01%–3.64%), significantly higher in ruptured (18.6%; 95% CI: 5.13%–36.26%) versus unruptured cases (0.23%; 95% CI: 0%–1.36%) (P < 0.01). Hemorrhagic complications occurred at 1% (95% CI: 0%–3.36%) pooled rate. Thromboembolic complications were reported at 6.1% (95% CI: 2.60%–10.73%), decreasing to 4.35% (95% CI: 1.91%–7.54%) after excluding one outlier study.
Limitations: Only five studies, some with small number of patients, were included in this meta-analysis which may limit the generalizability of our findings. The absence of long term follow-up also limits the assessment of treatment durability.
Conclusions: In this meta-analysis, we found that Vantage initial experience is similar to previous version of the Pipeline Embolization Device in terms of safety and efficacy for treatment of intracranial aneurysms, in particular unruptured aneurysms. Further prospective and comparative studies with patient outcome data specific to aneurysm location are needed to confirm the safety and efficacy of Vantage.
ABBREVIATIONS:
- CoNiCr
- cobalt-nickel-chromium alloy
- DFT
- drawn filled tube
- PED
- Pipeline Embolization Device
The Pipeline Embolization Device (PED; Medtronic) flow diverter marked a important advancement in aneurysm treatment, demonstrating effectiveness in achieving radiologic occlusion rates.1,2 This progress has been characterized by successive generations of PED devices.3⇓⇓-6 The PED Flex and the Pipeline Flex with Shield Technology aimed at improving maneuverability and thrombogenicity and paved the way for the latest device: the Pipeline Vantage Embolization Device (Vantage).7 The Vantage was introduced as a fourth-generation PED design, but it has some differences compared with previous models. While the PED Flex was composed of 48 wires made of 36 cobalt-nickel-chromium alloy (CoNiCr) and 12 platinum-tungsten wires (for radiopacity), the Vantage features, including a single-layer, self-expanding braided structure, and modified wire composition were made of CoNiCr with a drawn filled tube (DFT) of platinum for radiopacity. The number of wires was also modified and depends on the device size: 48 wires of CoNiCr with DFT platinum for the 2.5–3.5 mm diameter and 64 wires of mixed CoNiCr with DFT platinum (48 wires) and “pure” CoNiCr (16 wires) for the 4–6 mm diameter. Furthermore, like the previous generation, it also uses Shield Technology surface modification, possibly lowering the thromboembolic risk.6,8⇓-10
As intracranial aneurysm treatment progresses, it is essential to thoroughly evaluate the effectiveness and safety of emerging technologies. In this systematic review and meta-analysis, we sought to synthesize the existing evidence regarding the efficacy and safety outcomes of using the Vantage in treating intracranial aneurysms since its introduction in 2021.
MATERIALS AND METHODS
Literature Search
Following the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines,11 we conducted a comprehensive literature review on December 21, 2023. The AutoLit platform developed by Nested Knowledge was used for tasks such as deduplication, screening, and data extraction.
The search encompassed 4 major databases: PubMed, Web of Science, EMBASE, and Scopus. Customized search terms were formulated for each database, including variations of “aneurysm,” “pipeline,” and “vantage.” Detailed search terms formulated for each database can be found in the Supplemental Data. Additionally, a manual examination of references cited in the included studies was conducted to ensure comprehensive coverage.
Screening Process and Eligibility Criteria
Two co-authors independently reviewed the title of each article, abstract, and full text, resolving any uncertainties or ambiguities through consultation with a third senior coauthor.
Studies reporting treatment outcomes for intracranial aneurysms using the Vantage were included with no restrictions on publication date, country of origin, study design, aneurysm location, or rupture status. Non-English literature, case reports, case series with <5 eligible patients, conference abstracts, editorial comments, review articles, and irrelevant articles were excluded from the study.
Data Extraction
Two authors (P.V. and S.G.) independently collected data from each eligible study into an Excel 2021 spreadsheet (Microsoft), with a third author resolving any discrepancies.
Technical success was defined as the successful deployment and implantation of the intended Vantage device. Complete occlusion and adequate occlusion were defined according to specific criteria. For complete occlusion, O’Kelly-Marotta grade D and Raymond-Roy Occlusion Classification grade I were used as benchmarks. Additionally, for adequate occlusion, defined as complete aneurysm occlusion along with a neck remnant, O’Kelly-Marotta grades C and D, along with Raymond-Roy Occlusion Classification (grades I and II) were considered.12,13 These assessments were conducted during the last follow-up angiography for each aneurysm, ensuring standardized evaluation across the study.
Risk of Bias Assessment
The Risk of Bias in Nonrandomized Studies of Interventions (ROBINS-I; https://methods.cochrane.org/bias/risk-bias-non-randomized-studies-interventions) tool was used to assess the risk of bias in nonrandomized articles included in the meta-analysis.14 This tool covers 7 domains of bias, including confounding, selection of participants into the study, classification of interventions, deviations from intended interventions, missing data, measurement of outcomes, and selection of the reported result. The overall judgement about the risk of bias using this tool will be categorized as “low,” “moderate,” “serious,” or “critical.”
Statistical Analysis
All analyses were conducted in R software (Version 4.2.1; http://www.r-project.org/), using the “meta,” “metafor,” and “metasens” packages for the analytical tasks.15,16 Additionally, the robvis package (https://github.com/mcguinlu/robvis) was used for visualizing the risk of bias assessment results.17 Details of the review process and meta-analysis are provided in the Supplemental Data. Data on the number of participants and the outcomes of interest from each study were consolidated to conduct a proportion meta-analysis, aiming to synthesize the reported rates. Due to expected variability resulting from differences in institutional protocols, reporting methods, and follow-up techniques among the studies, we adopted a random-effects model for all meta-analytic processes.
For the analysis of proportional effect sizes, which do not follow a parametric distribution, we used the Freeman-Tukey double-arcsine transformation. Confidence intervals were estimated via the Wilson rank-based method.
The assessment of heterogeneity was performed using the I2 statistic and Q test. Heterogeneity was considered significant if I2 exceeded 50% or the P value for the Q test was <.05. In instances of marked heterogeneity, sensitivity analyses were undertaken to determine the influence of individual studies on the overall heterogeneity, identifying any outliers. Outlier and influence diagnostics were applied as outlined by Viechtbauer,15 using the InfluenceAnalysis function from the “dmetar” R package. If outliers were identified for any outcome, the meta-analysis was repeated excluding these studies to ensure the robustness of the conclusions.
Additionally, given the pre-established hypothesis regarding the potential impact of aneurysm rupture status on technical outcomes, subgroup analyses were performed for each outcome, categorized by the rupture status of the aneurysm.
Considering the limitations inherent in traditional funnel plot asymmetry tests for detecting publication bias in proportional meta-analyses, we opted for the Doi plot method to assess asymmetry and publication bias. The LFK index (https://ideas.repec.org/c/boc/bocode/s458762.html) was used to quantitatively evaluate the outcomes of this assessment. An LFK index between −1 and 1 suggested no asymmetry, while indices below −2 or above 2 indicated significant asymmetry. Values between −1 and −2, or 1 and 2, pointed to minor asymmetry.
RESULTS
Systematic Review and Meta-Analysis Findings
Screening and Selection of Articles.
Figure 1 illustrates the systematic selection process of studies according to PRISMA guidelines, as outlined in the Materials and Methods section. The search initially identified 57 records, which was reduced to 32 after the removal of duplicates. These records were then subjected to title and abstract screening, narrowing the field to 6 studies for full-text evaluation. Ultimately, 5 studies, encompassing a total of 373 participants, were deemed suitable for inclusion in the meta-analysis.5⇓⇓-8,18
PRISMA flow chart of the included studies. WoS indicates Web of Science.
Study Characteristics.
All the included studies were published in 2023 or 2022 and were conducted across various countries, including Germany, the UK, and Australia. Participant ages across the studies ranged from 50 to 60 years, with a predominance of female participants. The selection of studies was not contingent upon the site of the aneurysm, incorporating aneurysms from multiple origins, notably the ICA and the MCA as the most frequent sites. Additional specifics on the study parameters and aneurysm shapes of included cases are reported in the Supplemental Data.
Pre-, peri-, and postprocedural antiplatelet therapy varied among the studies. Although all studies used dual antiplatelet therapy for elective cases, the drugs, doses, and testing protocols differed in each study. Additionally, the periprocedural antiplatelet therapy protocol for ruptured aneurysms also varied among studies and in some cases among patients.
Notably, all studies reported a follow-up duration of 6–7 months, except for the study by de Villiers et al,6 which had a 3-month follow-up period. Among the studies, 2 specifically focused on cases of unruptured aneurysms,6,7 while the remaining 3 also included cases with ruptured aneurysms. Of these, Booth et al5 and Sciacca et al8 provided separate outcomes for both subgroups, which were analyzed in subgroup meta-analyses. The study by Vollherbst et al,18 not reporting outcomes separately for the 2 groups and predominantly involving unruptured aneurysm cases (>95%), was considered as mainly studying unruptured cases in the subgroup analyses.
The Supplemental Data present the outcomes of interest such as adequate and complete occlusion, technical success, additional coiling, and in-stent balloon angioplasty. Complications extracted encompassed thromboembolic and hemorrhagic events, along with total mortality. Moreover, while data on occlusion status during imaging follow-up and recanalization rates were extracted, these outcomes were not included in the meta-analysis due to the limited number of studies reporting these metrics.
Risk of Bias.
The methodologic quality of the included studies, assessed using the ROBINS-I tool, is depicted in Fig 2 as a traffic light plot. This evaluation revealed that most domains and studies exhibited low risk of bias concerns. However, moderate concerns were identified in 1 study8 regarding missing data, and another study6 demonstrated concerns related to bias in the measurement of outcomes.
Results of the methodologic quality assessment of the included studies based on the ROBINS-I tool.
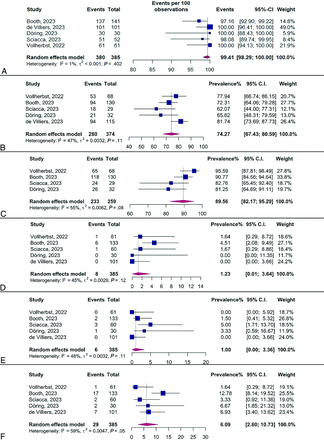
Technical Success.
As shown in Fig 3A, the meta-analysis reported a pooled rate of technical success at 99.41% (95% CI, 98.29%–100%), with no significant heterogeneity detected (I2 = 1%, P = .402). A further stratified analysis by rupture status, shown in the Supplemental Data, indicated a pooled rate of 99.54% (95% CI, 98.51%–100%) in the unruptured subgroup, compared with 100% (95% CI, 86.28%–100%) in the cohort of ruptured cases from Booth et al,5 without significant differences between groups (P = .871).
A, Forest plot of the random-effects proportion meta-analysis of the reported rates of technical success. B, Forest plot of the random-effects proportion meta-analysis of the reported complete occlusion rates. C, Forest plot of the random-effects proportion meta-analysis of the reported adequate occlusion rates. D, Forest plot of the random-effects proportion meta-analysis of the reported total mortality rates. E, Forest plot of the random-effects proportion meta-analysis of the reported rates of hemorrhagic complications. F, Forest plot of the random-effects proportion meta-analysis of the reported rates of thromboembolic complications.
Complete Occlusion.
Figure 3B illustrates the forest plot for a random-effects proportional meta-analysis pooling rates of complete occlusion. The median follow-up was 6 months (range, 3–7.1 months). This analysis yielded a pooled rate of 74.27% (95% CI, 67.43%–80.59%), with observed heterogeneity not reaching statistical significance (I2 = 47%, P = .11). The subgroup analysis, based on aneurysm rupture status and presented in the Supplemental Data, also found no significant differences (P = .72).
Adequate Occlusion.
Figure 3C displays the forest plot from a random-effects proportional meta-analysis examining reported rates of adequate occlusion. The pooled rate of adequate occlusion was determined to be 89.56% (95% CI, 82.17%–95.29%), with a moderate degree of heterogeneity observed (I2 = 55%, P = .08). This variability led to conducting a sensitivity analysis, the results of which are presented in the Supplemental Data. This analysis identified no studies as potential outliers. Additionally, a subgroup analysis, stratified by aneurysm rupture status and illustrated in the Supplemental Data, showed no statistically significant differences between groups (P = .80).
Total Mortality.
Figure 3D shows the forest plot for the meta-analysis of total mortality rates, with a pooled rate of 1.23% (95% CI, 0.01%–3.64%) and moderate heterogeneity (I2 = 45%, P = .12). The subgroup analysis, shown in the Supplemental Data, reports a mortality rate of 0.23% (95% CI, 0%–1.36%) in unruptured aneurysms and 18.58% (95% CI, 5.13%–36.26%) in ruptured cases, with significant differences between subgroups (P < .01). All studies reported a periprocedural mortality rate of zero.
Hemorrhagic Complications.
The meta-analysis of hemorrhagic complications is presented in Fig 3E, showing a pooled rate of 1% (95% CI, 0%–3.36%), with moderate heterogeneity (I2 = 48%, P = .11). The subgroup analysis based on aneurysm rupture status and illustrated in the Supplemental Data detected no significant differences in rates of hemorrhagic complications between groups (P = .15). These findings warrant cautious interpretation due to low statistical power and wide confidence intervals.
Thromboembolic Complications.
Figure 3F displays the forest plot from a random-effects meta-analysis on thromboembolic complications, revealing a pooled rate of 6.09% (95% CI, 2.60%–10.73%). Given considerable heterogeneity (I2 = 69%, P = .05), a sensitivity analysis was conducted, shown in the Supplemental Data, identifying the study by Booth et al5 as an outlier. The postexclusion analysis, depicted in the Supplemental Data, reported an adjusted pooled rate of 4.35% (95% CI, 1.91%–7.54%) with minimal heterogeneity (I2 < 0.5%, P = .42). A further analysis stratified by aneurysm rupture status, also in the Supplemental Data, reported a thromboembolic rate of 6.02% (95% CI, 2.74%–10.29%) in unruptured aneurysms and 10.64% (95% CI, 0.77%–26.36%) in ruptured cases and found no significant differences between subgroups (P = .19).
Publication Bias
The Supplemental Data 10 to 17 exhibit the Doi plots for each respective outcome as outlined in the Results section. These figures reveal significant asymmetry (LFK index > 2) for the outcomes of adequate occlusion, complete occlusion, and thromboembolic complications. This asymmetry suggests the potential presence of bias, which may be attributable to small study effects or publication bias.
DISCUSSION
This systematic review and meta-analysis provided evidence supporting the Vantage as a promising treatment option for intracranial aneurysms. It shows a high technical success rate, aneurysm occlusion, and relatively low mortality and complication rates.
The Vantage features a DFT wire structure with higher pore density in both 64- and 48-wire implants. This design, also found in recent flow diverters like the P64-MW-HPC (phenox), the Derivo Embolization Device (Acandis), and the Silk Vista (Balt), enhances the overall visibility of the struts.19⇓-21 Thinner wires and increased pore density compared with earlier models like the Pipeline Flex aim to improve blood stagnation within the aneurysm by giving a scaffold for neointimal formation at its neck and possibly leading to higher occlusion rates.18 Nonetheless, the current meta-analysis found a complete occlusion rate of 74.2% for intracranial aneurysms treated with the Vantage, similar to earlier PED versions and other flow diverters. The Prospective Study on Embolization of Intracranial Aneurysms with Pipeline Embolization Device (PREMIER) prospective study included aneurysms treated with the original PED or PED flex (without Shield Technology) and achieved a 81.9% complete occlusion rate at 1 year.22 The Safety and Clinical Effectiveness of Pipeline Shield Device for Intracranial Aneurysms in an Australian Cohort (SCOPE-AUS) study found that the PED with Shield Technology achieved 78.5% complete occlusion at 6 months and 92.5% at 18 months.23 Similarly, the PEDESTRIAN registry also with Pipeline Flex with Shield Technology showed a complete occlusion rate of 78.5% at 1 year.24 The clinical trials of Becske et al2 showed that the PED treatment resulted in 73.6% complete occlusion at 180 days, increasing to 95.2% at 5 years. Additionally, in the meta-analysis of Greco et al25 of 1952 aneurysms treated with various PEDs, complete occlusion rates were 75.5% and 76.5% in short- and long-term follow-ups.
Integration of the Shield Technology in the design of Vantage aimed to minimize its thrombogenicity.10 The current meta-analysis indicates a combined thromboembolic and hemorrhagic complication rate of 7.0%. Luo et al26 found a combined postoperative complication rate of 11.1% in their study on PEDs with Shield Technology for intracranial aneurysms. Variations in thromboembolic and hemorrhagic events across studies may stem from patient factors, aneurysm location, and anticoagulation protocols. Aggressive regimens may reduce clot formation but increase hemorrhagic risk. Although all were using dual antiplatelet therapy, none of the studies had an identical antiplatelet therapy protocol for the Vantage embolization. Differences in populations, follow-up periods, and methodologies among studies also influence the reported complication rates. Moreover, the Vantage smaller-wire diameters reduce device thickness, potentially lowering thrombotic risks and aiding endothelialization.27
Our findings showed significantly higher mortality rates in ruptured aneurysms compared with unruptured cases: 18.58% and 0.23%, respectively. Earlier publication on ruptured intracranial aneurysms treated with the PED reported mortality rates of 11.5%.28 This finding highlights the fact that even with the recent developments with PED, the Vantage is a good option for a limited number of indications within the ruptured case (ie, blister aneurysms). Moreover, periprocedural antiplatelet therapy poses challenges, especially in patients prone to hemorrhagic complications, underscoring the significance of considering rupture status in predicting mortality outcomes.29,30 The impact (positive or negative) of using single antiplatelet therapy with the Vantage in ruptured aneurysms on mortality remains unclear and requires further investigation. Unfortunately, due to the inclusion of only 5 studies with significant variability in antiplatelet protocols, this analysis could not be performed.
While providing valuable insights into the safety and effectiveness of the Vantage for intracranial aneurysms, this study acknowledges limitations. Some reviewed studies had small sample sizes, affecting generalizability and statistical power. Single-arm designs without control groups may introduce confounding factors. There were also some concerns about patient loss to follow-up in the included studies, without in most of them, robust measures to address this issue. Additionally, the absence of comprehensive demographic and baseline clinical data in studies makes it challenging to consider potential factors influencing outcomes at the individual aneurysm level. Moreover, caution is warranted when interpreting the results regarding the mortality rate of ruptured aneurysms, because it is not specified whether the mortality is procedure-related or secondary to complications from SAH. Finally, this study fails to report the rate of delayed braid deformation with the Vantage (not reported), which appears to be an emerging concern with new-generation flow diverters.31 Future studies should include it in their device evaluation, as recently recommended.32
CONCLUSIONS
This systematic review and meta-analysis on the initial experience with the Vantage suggests that it may be a promising treatment option for intracranial aneurysms. Rates of technical success, aneurysm occlusion, and adverse events are in line with rates in previous studies focusing on earlier versions of the PED, reflecting similar efficacy and safety. However, the increased mortality among cases of ruptured aneurysms warrants further investigation before expanding in this specific cohort. More multicenter prospective studies with larger samples are needed to better understand how patient and aneurysm characteristics may influence treatment outcomes.
Footnotes
J. Cortese, S. Ghozy, P. Valizadeh, and A. Hasanzadeh are co-first authors.
J. Cortese received educational grants from The French Society of Neuroradiology, the French Society of Radiology, the Philippe Foundation, and the Foundation Thérèse and René Planiol.
Disclosure forms provided by the authors are available with the full text and PDF of this article at www.ajnr.org.
References
- 1.
- 2.
- 3.
- 4.
- 5.
- 6.
- 7.
- 8.
- 9.
- 10.
- 11.
- 12.
- 13.
- 14.
- 15.
- 16.
- 17.
- 18.
- 19.
- 20.
- 21.
- 22.
- 23.
- 24.
- 25.
- 26.
- 27.
- 28.
- 29.
- 30.
- 31.
- 32.
- Received May 31, 2024.
- Accepted after revision September 20, 2024.
- © 2025 by American Journal of Neuroradiology