Abstract
BACKGROUND AND PURPOSE: This study investigates the practicality and utility of the “outline sign,” which refers to the thin curvilinear hyperenhancing line that may be seen along the margin of a meningioma on a spin-echo postcontrast T1-weighted image. For cases in which the differential diagnosis may include other tumors, visualization of the outline sign may help to increase the diagnostic confidence for a meningioma. Therefore, in the temporal bone region such as the cerebellopontine angle or jugular foramen, where differential considerations may include a schwannoma or paraganglioma, we additionally investigated whether the outline sign may be observed in these nonmeningioma lesions.
MATERIALS AND METHODS: A total of 39 clinical MRIs of meningiomas, schwannomas, and paragangliomas with confirmed histopathologic data were studied retrospectively. Two experienced head and neck radiologists independently assessed for the presence or absence of an outline sign and subsequently formed a consensus opinion while blinded to patient information and histopathologic data. Interreader reliability was assessed by Cohen κ statistics. Simple bivariate comparisons were performed on the consensus opinions to assess for statistical differences in presence of the sign in meningiomas versus schwannomas and paragangliomas. Sensitivity, specificity, and accuracy of the sign with respect to identifying an underlying meningioma were calculated.
RESULTS: Both readers displayed identical opinions in assessment of the outline sign in 34 of the 39 cases (87%), including 13 of the 14 meningiomas (93%), with substantial agreement (Cohen κ of 0.74). The outline sign was present in 12 of 14 meningiomas (86%), which was significantly greater in frequency compared with schwannomas (3 of 22, 14%) and paragangliomas (1 of 3, 33%). The outline sign demonstrated high sensitivity (86%), specificity (84%), and accuracy (85%) in identifying an underlying meningioma.
CONCLUSIONS: The outline sign can serve as a useful tool for diagnosing meningiomas. It may help distinguish meningiomas from other enhancing tumors, for example schwannomas and paragangliomas in the temporal bone region.
SUMMARY
PREVIOUS LITERATURE:
MR signal characteristics of the peripheral rim of meningiomas have been investigated in past decades. Among them, 2 studies reported observation of enhancement at the tumor periphery on contrast-enhanced T1-weighted imaging, supporting the theory of a rich capsule vascular supply rather than simply CSF space. Our study further investigated this rim enhancement phenomenon, which we termed the “outline sign,” and focused on its applicability in aiding the imaging diagnosis of meningiomas, and specifically in distinguishing meningiomas from schwannomas and paragangliomas when in the temporal bone region.
KEY FINDINGS:
The outline sign was noted in a majority of histopathologically histopathologically proved meningiomas, with substantial interreader agreement. It was noted much less frequently in schwannomas and paragangliomas. The outline sign demonstrated high sensitivity (85%), specificity (84%), and accuracy (85%) in identifying a meningioma.
KNOWLEDGE ADVANCEMENT:
The outline sign is a useful tool that can aid in the MRI diagnosis of meningiomas. In the temporal bone region, visualization of an outline sign can help increase the suspicion for a meningioma over a schwannoma or paraganglioma.
Meningiomas can occur intracranially and in the temporal bone region in various locations. They have imaging features that have been well described, including intermediate T2 signal, homogeneous enhancement (unless calcified), and presence of a dural tail on MRI, and adjacent bony hyperostosis on CT.1
When located in the temporal bone region, differential diagnostic considerations could include a vestibular schwannoma (when in the internal auditory canal and/or cerebellopontine angle cistern), or a lower cranial nerve schwannoma or jugular/jugulotympanic paraganglioma (when in the temporal bone region). To help distinguish between these entities, additional scrutiny for classic imaging features of schwannomas and paragangliomas is helpful. Schwannomas tend to be smooth lobulated lesions with fairly homogeneous enhancement, although they may have cystic components especially when large; they cause smooth expansile pressure scalloping of surrounding bone if they approach it. Paragangliomas enhance avidly, and may demonstrate internal flow voids due to their vascular nature; if they occur in regions surrounded by bone, as with a jugular or a jugulotympanic paraganglioma, the surrounding bone shows mottled, permeative changes.1
Although these imaging features are helpful, there may be cases when they are absent or equivocal, or there may be overlapping characteristics, rendering interpretation and diagnosis challenging. In this retrospective study, we investigate another imaging feature, which we termed the “outline sign.” We have observed that meningiomas often exhibit a thin hyperenhancing perimeter on contrast-enhanced T1-weighted MR images, particularly noticeable on a spin-echo T1-weighted MR sequence, resembling a thin wire fence around the border of a farm, or the outline drawn with a sharp coloring pencil around a picture before coloring in the center. A variation of this has recently been described in the literature; a few studies reported peripheral/capsular enhancement in meningiomas, but on postcontrast T2 FLAIR sequence.2⇓-4 At times, it may exhibit a serrated pattern due to surface CSF clefts.1
In this study, we aimed to determine the effectiveness of the outline sign as an imaging marker for meningiomas. For the particular scenario of the temporal bone region, we examined the applicability of the outline sign in aiding differentiation of meningiomas from schwannomas and paragangliomas.
MATERIALS AND METHODS
Study Selection
This was performed as a retrospective study. The methodology proposed in the Standards for Reporting of Diagnostic Accuracy Studies 2015 checklist was followed. Imaging reports for MRI examinations retrieved from the Massachusetts Eye and Ear PACS database between September 1, 2023 and December 31, 2023 were queried separately for the terms “meningioma,” “schwannoma,” and “paraganglioma”/“glomus”/“carotid body tumor.” This yielded a total of 270 distinct cases. Each full-length imaging report and the patient’s electronic medical records were then reviewed to confirm a history of pathology-proven meningioma, schwannoma, or paraganglioma, resulting in 44 histopathologically-proven cases. If the imaging study retrieved was performed subsequent to an antecedent surgical resection or intervention (e.g., biopsy), older imaging records were then reviewed to select the most recent preoperative or prebiopsy MRI, to ensure that imaging features of the original tumor were analyzed.
Exclusion criteria included absence of histopathologic confirmation, absence of preoperative imaging, absence of preoperative contrast-enhanced spin-echo T1-weighted sequences, and suboptimal image quality (e.g., motion degradation or hardware artifact). A final total of 39 cases (14 meningiomas, 22 schwannomas, and 3 paragangliomas) met all inclusion criteria and were included in our cohort (Fig 1).
Study design.
MR Imaging Acquisition
MRI studies were performed on 1.5 or 3T units. All 2D contrast-enhanced spin-echo T1-weighted sequences from whole brain, skull base, internal auditory canal, or neck MRs were analyzed. Axial and coronal T1 spin-echo postcontrast images ranged from 2 to 4 mm in slice thickness.
Outline Sign
The included cases were reviewed for the presence or absence of an outline sign. An outline sign was considered present if there was thin (≤1 mm), curvilinear hyperenhancement along the perimeter of the lesion on a postcontrast spin-echo T1-weighted sequence in any imaging plane. This thin hyperenhancing perimeter may be complete (seen continuously all around the border of the mass), or incomplete (seen intermittently in short segments all around the border of the mass, or seen continuously but only along a portion of the border). This outline may often demonstrate a serrated pattern, a term that alludes to small intermittent indentations or “clefts” along the border of the mass, precluding a smooth margin; when a serrated pattern was observed, this was recorded as well. Examples of the outline sign and serrated edges are shown in Fig 2. Examples of what do not constitute the outline sign are shown in Fig 3. Of note, adjacent dural enhancement or dural tail were not considered as representative of the outline sign.
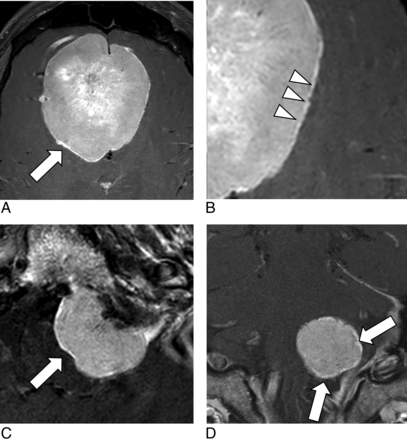
A, Contrast-enhanced axial T1-weighted image in a 46-year-old man demonstrates a large mass in the anterior cranial fossa continuing to the olfactory groove, which was subsequently resected and confirmed to be a meningioma. The mass demonstrates a thin rim of hyperenhancement (“outline sign”) that is especially prominent along its right posterolateral margin (arrow). B, A magnified view of this meningioma shows areas of rim enhancement (arrowheads) with a jagged appearance (“serrated edges”). Axial (C) and coronal (D) contrast-enhanced T1-weighted images in a 47-year-old with pathologically proven meningioma show an outline sign around the periphery of the lesion (arrows). Note the serrated pattern of this enhancing edge.
Potential pitfalls of the outline sign. A, Axial contrast-enhanced T1-weighted image in a 34-year-old with pathologically proven vestibular schwannoma shows small focal short/discrete (left arrow) or punctate/rounded/nodular (right arrow) areas of enhancement including some near the periphery (arrows). The short/discrete or punctate/nodular rather than thin curvilinear appearance is not consistent with the outline sign. B, Axial contrast-enhanced T1-weighted image in a 46-year-old with pathologically proven vestibular schwannoma. There is linear enhancement along the margin of the internal auditory canal (arrows), and it is difficult to determine whether this is intrinsic to the dural lining or if it is along the periphery of the tumor that may have cystic components at its lateral aspect. However, as this linear enhancement reaches the cerebellopontine angle cistern (arrowheads), it appears to continue along the posterior petrous ridge away from the tumor, favoring the former theory of being related to the dura. As such, this is equivocal for an outline sign. C, Axial contrast-enhanced T1-weighted image shows a pathologically proven left vestibular schwannoma in a 49-year-old patient. The central portion of the tumor shows diminished enhancement within areas of focal cystic change, falsely exaggerating the degree of mural enhancement (arrows). This enhancing periphery is thicker than 1 mm and does not have the thin appearance of the outline sign.
Image Review
Two head and neck radiologists at Massachusetts Eye and Ear with 7 years (K.L.R.) and 28 years (L.V.R.) of experience underwent a group training period to develop a consistent and reproducible technique for identifying the outline sign. They then independently reviewed a randomized, anonymized list of these 39 cases to determine the presence or absence of an outline sign. The readers also recorded the presence or absence of a serrated pattern to the outline sign. The readers were blinded to the original radiology reports, patient demographics, clinical information, histopathologic data, and the other reader’s findings. Subsequently, the 2 readers reviewed discrepant cases together for consensus opinion.
Statistical Analysis
Interreader reliability was evaluated based on Cohen κ statistics for assessment of imaging characteristics (outline sign and serrated edges) between the 2 readers.
The consensus data set was used for additional statistical analysis. Simple bivariate comparisons were performed to assess the association between tumor and imaging characteristics. This was done by using Fisher exact test. Additionally, the tumor type was dichotomized to meningioma versus nonmeningioma groups and simple bivariate comparisons were repeated. Sensitivity, specificity, and accuracy of the outline sign in predicting final tumor histopathology (meningioma, schwannoma, or paraganglioma) were calculated.
All statistical analyses were performed by using R Version 4.2.3 (R Core Team, 2023) and 2-tailed P value less than 0.05 was used to determine statistical significance.
RESULTS
Clinical data regarding patient demographics and tumor location are presented in Table 1.
Clinical features of meningiomas, schwannomas, and paragangliomas
Both reader A and reader B independently identified the outline sign in a high percentage of meningiomas, as shown in Table 2 (86% and 93%, respectively). There was interreader agreement on the presence or absence of the outline sign in 34 of the 39 cases (87%), notably agreeing in 13 of the 14 meningiomas (93%). Findings yielded a Cohen κ index of 0.74, compatible with substantial agreement between readers. Interreader agreement in assessment of the presence or absence of serrated edges was lower, yielding a Cohen κ index of 0.49, suggesting only moderate agreement.
Reader assessments on presence of outline sign performed independently and as a consensus opinion classified by tumor histopathology
After reviewing discrepant cases by consensus opinion, an outline sign was identified among 12 (86%) of the 14 meningiomas (Table 3). The sign was identified far less frequently among schwannomas and paragangliomas, in only 14% (3 of 22) of schwannomas and 33% (1 of 3) of paragangliomas. These differences were found to be statistically significant (P < .001). Serrated edges were seen in only 57% of meningioma cases, which was still more frequently seen than in schwannomas (9%) and paragangliomas (33%) (P = .0158). These differences were also found to be statistically significant (P = .0158).
Presence of outline sign and/or serrated edges (meningioma versus other tumor types) following consensus read
The presence of an outline sign as a diagnostic marker for meningioma shows promise, yielding a sensitivity of 86%, specificity of 84%, and accuracy of 85%.
DISCUSSION
In this study, we evaluated the utility of the outline sign, defined as the presence of any amount of thin, curvilinear hyperenhancement along the perimeter of the tumor on a postcontrast spin-echo T1-weighted sequence, as a diagnostic marker for meningioma. Second, we selected the temporal bone region as an anatomic site for specific examination of the applicability of the outline sign in helping to distinguish meningiomas from other tumors that may arise in this area, such as schwannoma and paraganglioma. Assessment of the presence of this sign by 2 experienced head and neck radiologists showed substantial interreader agreement, suggesting that it can be reliably identified on MRI when present, rendering it suitable for practical application. This thin enhancing peripheral line may occasionally have a serrated pattern.
Our data suggest that the outline sign is highly indicative of a meningioma, which when seen would favor the diagnosis of a meningioma much over a schwannoma or a paraganglioma, should those be within differential diagnostic consideration based on anatomic location. Fig 4 is an example of an enhancing lesion in the right cerebellopontine angle/internal auditory canal that was thought to be a schwannoma based on MRI, given its location and shape, but was subsequently proven to be a meningioma on histopathology. Review of the postcontrast T1-weighted images in retrospect suggested presence of an outline sign.
Contrast-enhanced axial T1-weighted image in a 40-year-old shows an enhancing lesion in the right cerebellopontine angle extending into the right internal auditory canal. The lesion was reported as a vestibular schwannoma by the interpreting radiologist. Histopathology upon resection revealed a meningioma. In retrospect, an incomplete rim of enhancement in keeping with an outline sign (arrow) is visible.
The MR signal characteristics of the peripheral rim of meningiomas have been a subject of investigation in prior studies. Several studies focused primarily on observations made on T2-weighted or FLAIR sequences.2⇓⇓⇓-6 In 2014, Enokizono et al5 evaluated rim patterns of intracranial meningiomas on 3T MRI on nonenhanced and contrast-enhanced 3D FLAIR imaging, correlating signal intensity to surgical cleavability and histologic tumor grading. They noted that a more prominently visible rim on contrast-enhanced 3D FLAIR suggested prominent pial vascular supply to the tumor. A retrospective study by Panyaping et al4 in 2023 revealed rim enhancement on contrast-enhanced 3D FLAIR to be highly sensitive (89.2%), highly specific (93.5%), and the most accurate (90.2%) for a meningioma among their other studied imaging markers of interest, including presence of a dural tail, marrow edema, hyperostosis, and homogeneous tumoral enhancement.
A few studies included observations made on postcontrast T1-weighted imaging. In 2003, Oguz et al2 studied 30 meningiomas on 0.5T MRI and examined T2-weighted fast spin-echo, T1-weighted spin-echo sequences, and fast FLAIR sequences before and following contrast administration, noting 21 with rim enhancement on fast FLAIR and only 1 on the spin-echo sequences. They suggested that this pattern may help to differentiate meningiomas from other extra-axial masses. That same year, Takeguchi et al6 examined the brain-meningioma interface of 50 meningiomas on 1T MRI, 41 of which were histologically proven. They noted FLAIR hyperintensity and T1-weighted postcontrast enhancement in a most of lesions, and concluded that the rim did not reflect a CSF cleft, but instead might reflect the capsule structure at the tumor surface. In 2016, Uchida et al7 focused on signal characteristics of the rim on T2-weighted sequence on 3T MRI in an effort to decipher the histologic correlate for this finding. They examined 53 meningiomas, 22 of which underwent histopathologic analysis. They reported that of the 53 tumors, 37 showed a T2-hyperintense rim, of which 28 showed postcontrast T1-weighted enhancement at the brain-meningioma interface and therefore did not reflect CSF space, and rather was postulated to reflect a microvascular-rich capsule layer, endorsing the findings of Takeguchi et al6 11 years earlier.
Our study focused primarily on signal characteristics of the periphery of meningiomas on contrast-enhanced spin-echo T1-weighted imaging and its applicability as a potential MR imaging marker for meningiomas. We found more intense enhancement around the peripheral margin of a meningioma compared with the main bulk of the tumor with high sensitivity and specificity. Given that spin-echo postcontrast T1-weighted imaging is a staple of MRI protocols at most institutions, scrutinizing MR features of a mass lesion for this imaging sign can readily be adopted in practice with existing imaging protocols, without the need for modifying protocols and/or adding extra sequences.
This phenomenon of rim enhancement in meningiomas has been attributed in the past to their dual arterial supply.2,4,5 Meningiomas are predominantly supplied by meningeal arteries, which supply the central portion of the tumor, generating a characteristic sunburst pattern on angiography. The relative hyperenhancement along the margins of the tumor is thought to be related to a separate arterial supply from pial vessels to a microvascular-rich tumor capsule. Presence of rim enhancement has been shown to be more common in meningiomas demonstrating visible pial arterial supply on digital subtraction angiography.5 Furthermore, this rim enhancement may be associated with the presence of increased microvascular density within the tumor capsule based on prior studies with histopathologic correlates.6,7 Indeed, the studies by Takeguchi et al6 and Uchida et al7 showed differences in MR enhancement characteristics between the rim and the bulk of the tumor that may reflect a hypervascular tumor capsule.
Limitations of this study include its retrospective nature and in the number of included imaging studies due to the criterion of requiring that there be histopathologic confirmation to serve as diagnostic gold standard. The imaging protocol was not identical for all included MRI studies, as each examination was tailored to its specific clinical area of interest. However, this in fact simulates the actual global radiology practice landscape where scanners and imaging parameters vary between institutions and facilities, and lends confidence to practical application of the sign in real life due to robustness of this imaging sign across variations in scanners and scan techniques.
We investigated the utility of the outline sign in aiding imaging diagnosis of meningiomas independent of tumor location; as such we included meningiomas in all locations within our study cohort. Second, we sought to apply this to the specific scenario of the temporal bone region, focusing on locations where schwannoma and/or paraganglioma may be reasonable differential diagnostic considerations based on tumor site. We investigated whether the outline sign may positively contribute to a correct diagnosis at these sites. With a focus on the temporal bone region and with the inclusion criterion of an available histopathologic diagnosis, an added limitation is the low number of cases of histopathologically proven meningiomas, schwannomas, and paragangliomas specific to this location. This is in part related to an overall low surgical resection rate or biopsy rate, given extreme caution exercised when balancing the advantages of having a definitive histologic diagnosis against the downsides of potential damage to cranial nerves and vital function. Many are surveilled or radiated at times solely based on the degree of diagnostic confidence from imaging evaluation. Nonetheless, we thought it useful to study the cases available while ensuring satisfactory statistical power. In addition, the crucial role of imaging assessment informing clinical management highlights the necessity for increasing the radiologist’s armamentarium of imaging diagnostic tools to increase our diagnostic confidence. Although this study did not exclusively examine cases in these locations in order to maintain a sufficient sample size, we believe our results are applicable to the differentiation of these 3 tumor types in the temporal bone region.
A few potential pitfalls were identified in the use of the outline sign by reviewing cases in which independent reader assessments were discordant. Linear enhancement along the tumor capsule in 1 dimension may appear as punctate enhancement in an orthogonal dimension. Lesions in close proximity to bone may result in a false-positive outline sign, possibly because areas of marginal enhancement may be related to the adjacent dura or dural-osseous interface rather than the peripheral portion of the tumor. Cystic changes within a tumor may result in diminished central enhancement and residual solid enhancement around the periphery, potentially leading to a false-positive thicker “pseudo-outline.” In fact, this may potentially represent the “peritumoral halo” that has been described with schwannomas, where a rim of hyperenhancement is thought to reflect gadolinium extravasation along the margins of the tumor.8 This is distinct from the outline sign, which is thin and is thought to reflect enhancement of a tumor capsule.
CONCLUSIONS
The outline sign refers to thin curvilinear enhancement along the periphery of a meningioma that may be seen on spin-echo postcontrast T1-weighted sequence. It is a practical and helpful imaging feature that can aid in the diagnosis of meningiomas. In the temporal bone region, such as the cerebellopontine angle or jugular foramen region, it is an added imaging feature that may help to distinguish meningiomas from other enhancing tumors such as schwannomas or paragangliomas. This finding is thought to reflect enhancement of a hypervascular meningioma tumor capsule.
Footnotes
Disclosure forms provided by the authors are available with the full text and PDF of this article at www.ajnr.org.
References
- 1.
- 2.
- 3.
- 4.
- 5.
- 6.
- 7.
- 8.
- Received June 18, 2024.
- Accepted after revision July 23, 2024.
- © 2025 by American Journal of Neuroradiology