Abstract
BACKGROUND AND PURPOSE: Normal pressure hydrocephalus (NPH) is a diagnostic challenge because its clinical symptoms and imaging appearance resemble normal aging and other forms of dementia. Identifying NPH is essential so that patients can receive timely treatment to improve gait distortion and quality of life. An automated marker of NPH was developed and evaluated on clinical CT images, and its utility was assessed in a large patient cohort.
MATERIALS AND METHODS: A retrospective review was conducted of CT images from 306 tap test–responsive patients with NPH between January 2015 and January 2022. Control CT images were obtained from patients in the emergency department who were evaluated for headache and had unremarkable CT findings between June 2021 and August 2022. The ventricle-to-subarachnoid volume ratio (VSR) was automatically calculated by the imaging software and used as a predictor of NPH in linear regression modeling with adjustment for age and sex. The correlations of VSR with age, sex, and the receiver operating characteristic were computed.
RESULTS: VSR was significantly greater in patients with NPH than controls (P < .001). Importantly, VSR was not significantly correlated with age (P = .56, R2 = 0.001). VSR identifies NPH with a sensitivity and specificity of 94.1% and 92.5%, respectively, with an area under the receiver operating characteristic curve of 0.99 (95% CI 0.975–0.995).
CONCLUSIONS: Automated assessment of the VSR on head CT images identified probable NPH with 93% accuracy. The assessment of a large cohort of patients with NPH supports the generalizability of clinical screening of CT images. Moreover, the results support the utility of ventricle-to-sulcal concordance often used by radiologists but not currently a part of the accepted guidelines for imaging markers of NPH.
ABBREVIATIONS:
- AD
- Alzheimer’s disease
- AUC
- area under the curve
- AUROC
- area under the receiver operating characteristic
- ICV
- intracranial volume
- NIfTI
- Neuroimaging Informatics Technology Initiative
- NPH
- normal pressure hydrocephalus
- ROC
- receiver operating characteristic
- VP
- ventriculoperitoneal
- VSR
- ventricle-to-subarachnoid volume ratio
SUMMARY
PREVIOUS LITERATURE:
Normal pressure hydrocephalus (NPH) is a prevalent and debilitating disease. Fortunately, surgical shunting can provide significant benefits. Early detection is crucial but challenging because ventriculomegaly and the symptoms associated with NPH are nonspecific. Although automated assessments show promise for detecting NPH, the previous reliance on MRI limits their widespread availability for patient care, necessitating new approaches for analyzing routine clinical images. Ventricle-to-sulcal concordance is commonly evaluated by radiologists to differentiate NPH from other causes of ventriculomegaly, but Evan’s index and callosal angle are crude and indirect approximations. An automated direct measurement of ventricle-to-sulcal concordance for CT is needed.
KEY FINDINGS:
Our CT biomarker for NPH based on ventricle-to-sulcal concordance, ventricle-to-subarachnoid volume ratio (VSR), was a significant predictor of NPH (R2 = 0.76; P < .001; area under the curve = 0.99) in a cohort of 306 patient with NPH and 294 control patients. A VSR >2 classified NPH with high sensitivity (94.1%) and specificity (92.5%).
KNOWLEDGE ADVANCEMENT:
We developed an automated assessment of VSR and evaluated its efficacy as an initial screening tool for NPH. This large study supports ventricle-to-sulcal concordance as a valid clinical approach. Flagging routine CT head examinations with a VSR >2 can help more patients receive an earlier neurologic evaluation of NPH.
Normal pressure hydrocephalus (NPH) is a form of communicating hydrocephalus that leads to the gradual progression of gait impairment and increases the risk of falls.1 NPH may also cause cognitive dysfunction and urinary incontinence, resulting in the well-known triad of symptoms common to NPH. The prevalence of NPH increases with age and affects an estimated 6%–9% of those older than 80 years.2,3 Fortunately, NPH can be treated with the surgical placement of a ventricular shunt from which patients experience marked and long-lasting physical, mental, and social benefits.4⇓-6
There is a pressing need for early detection and better tools to screen for NPH. It is difficult to recognize NPH on the basis of clinical features alone because the symptoms are common among older adults.7 Radiographically, enlarged ventricles are nonspecific and can also be due to age-related atrophy or neurodegenerative disease. Furthermore, NPH should ideally be diagnosed before the complete triad of symptoms arises to achieve the best shunting response because the potential for functional recovery diminishes as the disease progresses.8⇓-10
Imaging serves a crucial role by providing the first indication that a patient might have NPH. A systematic detailed evaluation of a panel of radiologic markers is reported to achieve a sensitivity of 100% and specificity of 96% in identifying NPH,11 but these might not be assessed if there is not already a suspicion of NPH. Automated image analysis can serve as an objective, reproducible tool for the identification of NPH,12 but these analyses require specialized MRI sequences. Therefore, we developed an algorithm to identify NPH from clinical imaging of the head.
The intent of our retrospective study was to evaluate the efficacy of a method for automated NPH screening and not to replace verification of shunt-responsive NPH by using the tap test. We based our approach on the common radiologic practice of assessing the ventricle-to-sulcal concordance, thereby distinguishing NPH ventriculomegaly from age-related ventriculomegaly. Thus, we developed a marker derived from clinical CT examinations of the head that quantifies the lateral ventricle-to-supratentorial subarachnoid volume ratio, referred to as the ventricle-to-subarachnoid volume ratio (VSR). We tested the ability of our automated VSR to distinguish 306 tap test–responsive patients with NPH treated in the multidisciplinary Barrow Neurological Institute Normal Pressure Hydrocephalus Clinic from 294 control patients.
MATERIALS AND METHODS
Patients
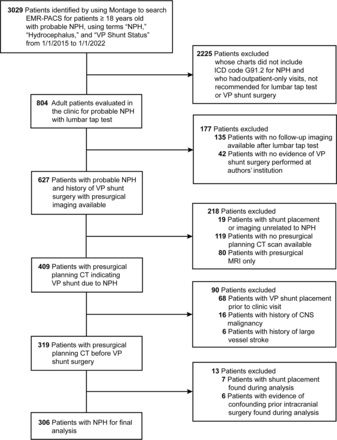
Study approval was obtained from the St. Joseph’s Hospital and Medical Center institutional review board for a retrospective search of patient electronic medical record-PACS by using Nuance mPower Montage (Nuance Communications, Burlington, Massachusetts), with a waiver of patient informed consent for the creation and evaluation of a deidentified data set. We identified a consecutive series of 306 patients with NPH from the Barrow Neurological Institute Normal Pressure Hydrocephalus Clinic who had presurgical planning CT studies and positive CSF tap tests from January 1, 2015, to January 1, 2022. The inclusion criteria were 18 years of age or older with probable NPH, searched by using the terms “NPH,” “Hydrocephalus,” or “VP shunt status,” which yielded 3029 patients. Individuals were excluded if they did not have an International Classification of Disease code G91.2 for NPH, if patient visits were conducted only outside of our institution, or if a lumbar tap test and ventriculoperitoneal (VP) shunt surgery were not recommended. There were 804 patients with probable NPH who received a tap test. Of these, 177 had no post-tap test imaging available or no evidence of VP shunt surgery performed at our institution, suggesting that they were lost to follow-up, had imaging or surgery outside of Barrow, or had a negative tap test result. Patients were then excluded if shunt placement was unrelated to NPH, they did not have presurgical planning CT or only had presurgical MRI, the shunt was placed before the clinic visit, there was a history of a large vessel stroke or CNS malignancy, or there was evidence of confounding earlier intracranial surgery (Fig 1). Probable NPH was diagnosed in our clinic by using established guidelines that require a combination of clinical examinations, brain imaging confirming ventriculomegaly, and responsiveness to objective gait testing measures after the removal of 30–40 mL of CSF. Control images were obtained from 294 patients presenting to the emergency department with headache who underwent CT imaging that was negative for acute intracranial findings from June 1, 2021, to August 1, 2022. Controls were selected from an original cohort of 600 cases to best match the skewed age and sex of the NPH cohort, although precise matching was feasible only for younger patients.
Patient inclusion and exclusion criteria. EMR-PACS = electronic medical record-PACS; ICD = International Classification of Disease. Used with permission from Barrow Neurological Institute, Phoenix, Arizona.
CSF Tap Test Protocol
Each patient underwent objective gait testing in the laboratory before and after the CSF tap test procedure. When most measures indicated improved gait and balance within 24 hours after the CSF tap test compared with baseline, the result of the CSF tap test was considered positive.
Head CT Imaging Technique
All participants had noncontrast head CT with 1.2-mm thin slice images obtained with the high spatial frequency bone algorithm. Patients with NPH underwent CT imaging after a lumbar puncture test as part of surgical planning for shunt placement. Control CT images were acquired as part of an institution-wide emergency department protocol. Twenty 5-mm thick slice images from either cohort were also analyzed to compare input methods.
Head CT Image Analysis
A CT image analysis was performed by using customized sequences that built upon routines and recommendations graciously detailed by Muschelli13 and Cauley et al.14 The automated program ran successfully on every patient examination that did not include intracranial hardware, CNS malignancy, or encephalomalacia from stroke, trauma, or intracranial surgeries. Briefly, DICOM files were anonymized and converted to Neuroimaging Informatics Technology Initiative (NIfTI) files by using MRIcroGL, version no. v1.2.20220720 (University of South Carolina, McCausland Center for Brain Imaging).15 The 2 cohorts were processed as a single group with our automated program. Analyses of NIfTI files were then performed with scripts by using the Functional Magnetic Resonance Imaging of the Brain (FMRIB) Software Library.16 Bone and fat were removed by using thresholding of voxels with values below 0 or above 120. The FMRIB Software Library’s Brain Extraction Tool (http://fsl.fmrib.ox.ac.uk/fsl/fslwiki/BET)17 was used for skull stripping. FMRIB’s Automated Segmentation Tool (http://fsl.fmrib.ox.ac.uk/fsl/fslwiki/fast)18 was used for image intensity inhomogeneity correction.14 CSF masks were obtained by further thresholding with a cutoff of 20. Using the FMRIB Linear Image Registration Tool (https://fsl.fmrib.ox.ac.uk/fsl/fslwiki/FLIRT),19,20 the extracted brains were registered to a standard brain atlas,21 and a mask was applied to obtain segmentation of supratentorial CSF (Fig 2). Two separate lateral ventricle atlases were applied to the segmented CSF by using our automated program to target the spectrum of unique features in normal ventricles and in ventricles enlarged by NPH. The standard ventricle atlas was able to conform to the gracile aspects of a normal ventricle, and the custom ventriculomegaly atlas (created from patients with probable NPH and atrophy who are not included in this study) was able to conform to the extreme ventricles in NPH. Both masks were applied to every patient, and the ventricle extracted by using a standard ventricle atlas was combined with the ventricle extracted by using the custom ventriculomegaly atlas to create a complete final lateral ventricle. The ventricle was then smoothed to remove holes, and a cluster analysis was performed to select only the largest intact clusters of the segmented lateral ventricle. Preprocessing and segmentation, on average, required 22.9 and 65.5 seconds, respectively, for each patient examination. We visually assessed the final ventricle segmentation for every patient to ensure >95% ventricle segmentation accuracy. The subarachnoid CSF volumes were determined by subtracting the lateral and third ventricle volume from the supratentorial CSF volume (Fig 2). The ventricle-to-subarachnoid ratio is, therefore, an approximation of ventricle-to-sulcal concordance but with boundaries that are readily identifiable on CT of the head. The code for the automated program is available upon request, and inquiries can be directed to the corresponding author for further details.
Stepwise segmentation of supratentorial CSF, shown in red, and the lateral ventricles, shown in green, to generate the VSR. Used with permission from Barrow Neurological Institute, Phoenix, Arizona.
Statistical Analysis
The diagnostic accuracy of the VSR was assessed by using nominal logistic regression to predict NPH group status on the basis of VSR with adjustment for age and sex. The level of significance was set at .05 by using 2-tailed tests. For comparison, the ventricle-to-intracranial volume (ICV) ratio was also calculated and evaluated by using the same nominal logistic regression with adjustment for age and sex. Additionally, Bland-Altman analysis was performed to evaluate the automated program if standard thick slice images are the desired input. No other reference standard was applicable, given the nature of this study and the intent to use this tool in conjunction with the tap test. Results were explored by using receiver operating characteristic (ROC) curves generated with JMP statistical software (JMP Statistical Discovery) to evaluate sensitivity and specificity. Additional evaluation of the test characteristics of the VSR to identify probable NPH status was then performed by using the Model Classification Explorer (JMP Add-In: available at Exploring Model Classification Thresholds - JMP User Community) to determine the threshold cutoffs that maximize sensitivity and specificity, and minimize the misclassification rate. Mean group differences were evaluated by using the 2-tailed, 2-sample Student t test. Based on preliminary power analysis and earlier research, it was determined that 30 patients with NPH and 30 control patients would be a sufficient sample size to reliably differentiate the 2 groups by using a ratio of ventricle to ICV. Our study was intentionally designed to be overpowered, with an ample sample size to thoroughly assess VSR, thereby enhancing the study’s overall generalizability.
RESULTS
The characteristics of the study cohort are shown in the Table. The mean ages ± standard deviations for patients with NPH and control patients were 76 ± 7 and 74 ± 8 years, respectively. Among the 306 patients with NPH, 195 were men (64%), and 111 were women (36%). In the control group, 169 of 294 were men (57%), and 125 were women (43%). The VSR for men versus women was 0.91 versus 0.75 in the control group (Online Supplemental Data, P = .04, size effect =0.015). Although the NPH group was slightly older, there was not a statistically significant increase in the VSR with age in the control group (Online Supplemental Data, R2 = 0.001, P = .56, size effect =0.001). Older age was significantly correlated with increased ventricle to ICV ratio (P < .001; R2 = 0.19).
Cohort characteristics by group
Logistic regression analysis adjusted for age and sex showed that the VSR was a significant predictor of NPH status (overall model fit R2 = 0.76, P < .001) with an overall model area under the receiver operating characteristic (AUROC) curve of 0.985. Logistic regression for the NPH group status without adjustment for age or sex with VSR as a predictor also yielded R2 = 0.76, P < .001, and AUROC curve of 0.985. For comparison, logistic regression adjusted for age and sex was also analyzed with the ventricle to ICV ratio as a predictor, which also predicted NPH (P < .001), although the overall model was less predictive (R2 = 0.50 and the AUROC curve of 0.932). Logistic regression for ICV as a predictor without adjustment for age and sex yielded a similar result (R2 = 0.49, P < .001 and AUROC curve of 0.929).
Fig 3 shows a threshold analysis of VSR between patients with NPH and patients in the control group. A VSR with a threshold of greater than 2 yielded optimal statistically significant separation and classification of NPH (Fig 3A). Fig 3B shows the VSR ROC with area under the curve (AUC) of 0.99 (95% CI 0.975–0.995) and ventricle to ICV ratio ROC with an AUC of 0.93 (95% CI 0.91–0.95).
Threshold analysis comparing the VSRs of patients with NPH and control patients. A, A threshold of 2 optimally separated NPH from control patients. A total of 18 of 306 (5.9%) patients with NPH and 22 of 294 (7.5%) control patients were misclassified. B, The AUROC curve for VSR was 0.99 with a 95% CI between 0.975 and 0.995 compared with that of the ventricle to ICV, which was 0.93 (95% CI 0.91–0.95). Used with permission from Barrow Neurological Institute, Phoenix, Arizona.
Of the 306 patients with NPH, 18 (5.9%) were misclassified as normal, and of the 294 control patients, 22 (7.5%) were misclassified as having NPH. The sensitivity and specificity of this ratio were 94.1% (95% CI 90.9%–96.2%) and 92.5% (95% CI 88.9%–95.0%), respectively.
DISCUSSION
We demonstrate the efficacy of an automated tool to assess the VSR on clinical CT head examinations. The results support its utility as an initial screening tool for NPH. After adjusting for age and sex, a higher VSR was predictive of NPH (P < .0001, AUROC curve =0.99), with each unit increase associated with 9-fold increased odds of NPH. With a VSR cutoff of 2, the sensitivity and specificity were 94.1% and 92.5%, respectively. These results suggest that patient age has a minimal effect on VSR for predicting NPH. The logistic regression results underscore these findings.
This analysis is one of the largest automated clinical CT imaging analyses to demonstrate highly accurate identification of NPH compared with control patients. We based our technique on the concept of ventricle-to-sulcal concordance. Radiologists commonly distinguish NPH from age-related atrophy by crudely assessing ventricle-to-sulcal concordance with Evans index and indirectly with callosal angle; however, a direct measurement is not a formal part of the NPH diagnostic guidelines.22,23 Age-related atrophy tends to expand the space inside and outside the brain in a similar fashion, maintaining concordance.24 In practice, radiologists often look for this expansion at the sulci, although the entire subarachnoid volume is expanded. Our findings support the validity of ventricle-to-sulcal concordance as a clinical approach.
Automated assessments can provide a valuable tool for detection and early recognition of NPH. Among the patients in the Open Access Series of Imaging Studies and the Alzheimer Disease Neuroimaging Initiative databases, 12.4% of patients had a callosal angle measurement that met imaging criteria for probable NPH.25 Although MRI is the standard for high resolution segmentation and our approach does not segment white and gray matter, in order for NPH measurements to be widely available for patient care, we recognized that new approaches are needed for the analysis of routine clinical images, such as CT rather than MRI. In future work, we plan to adapt the previously published automated callosal angle program25 for analysis of ventricles derived from clinical CT images to increase its specificity and follow our current clinical assessment pathway.26 We also suggest that once a physician suspects a patient might have NPH as indicated by automated programs, it is feasible to conduct additional radiologic11 and clinical2 screening because this automated program may include patients with non-NPH ventriculomegaly. These patients can be further assessed to identify those who may benefit from a detailed neurology expert consultation and a possible confirmatory tap test for shunt-responsive NPH.
The VSR has advantages over the ventricle to ICV ratio, which is more affected by age-related atrophy than the VSR.27 In our control group, age explained approximately 19% of the variance in the ventricle to ICV ratio but only 0.1% of the variance in the VSR. The VSR was slightly more predictive of NPH status within the group, with an AUROC curve of 0.99 compared with 0.93 for the ventricle to ICV ratio.
A limitation of our data is that we did not calculate the VSR in non-NPH ventriculomegaly, such as Alzheimer’s disease (AD). Although it is possible that a VSR >2 includes patients with ventriculomegaly secondary to neurodegenerative disease, current work suggests sufficient differentiation of NPH and AD with ventricle volume alone and ratios like ours on MRI.12,28 In fact, there is a growing pool of data suggesting that the 2 conditions are not mutually exclusive, but there are mixed results on the benefits of treating NPH with comorbid AD. One study demonstrated that patients being evaluated for NPH had a greater improvement in post-tap test cognition and gait if they also had positive CSF biomarkers and clinical features of AD compared with patients without such features of AD.29 Other studies suggest no change in shunt response, or, in moderate-to-severe AD, a postshunt decline in cognition for patients with NPH and AD.30,31 Nonetheless, the consensus is that the presence of comorbid NPH and AD should not exclude patients from NPH treatment,29⇓⇓-32 and thus, referral to an NPH clinic when a patient has a VSR >2. The goal of the present study was to validate VSR in tap test–responsive patients with NPH so that in future work we can correlate VSR to pre-tap and post-tap test gait data, amyloid/τ CSF studies, and response to shunting. Second, the skew toward younger female patients in our control population resulted in a small but statistically significant difference in age compared with the NPH group. From an original cohort of 600 control cases, we were able to have precise matching at the younger end of our age range, then we adjusted for age and sex in our final analysis to address the residual difference. Another limitation is the use of 1.2-mm thin slice images, instead of the more commonly archived thick slice images. To address this issue, we compared input methods in a small subset of patients with a Bland-Altman analysis. Although there was a significant bias between methods, the correlation was strong, and a conversion could be applied to maintain diagnostic accuracy. Nonetheless, further optimization of the automated program will be required to input thick slice images. Finally, there is a distinct and related appearance of NPH with superior subarachnoid space narrowing called disproportionately enlarged subarachnoid space hydrocephalus.33 Although we do not screen for it in the current version of our program, we plan on evaluating suprasylvian subarachnoid volumes in future work because they may be more specifically attenuated in NPH.34
CONCLUSIONS
Automated assessment of VSR is a noninvasive, feasible, and objective method with high clinical utility for NPH screening in patients undergoing head CT. We report high sensitivity and specificity in a large cohort of 306 individuals identified as having tap test–responsive NPH on the basis of evaluations at a multidisciplinary normal pressure hydrocephalus clinic and objective gait improvement after tap testing, which supports the study’s generalizability. We are working to implement this screening process into the clinical workflow at our institution. By incorporating VSR into the standard practice for diagnosis of NPH, we hope patients are evaluated and treated earlier to avoid this reversible form of dementia and its associated risks.
Acknowledgments
We thank the staff of Neuroscience Publications at Barrow Neurological Institute for assistance with manuscript preparation.
Footnotes
The Rudi Schulte Research Institute and Barrow Neurological Foundation provided funding support to conduct this research.
Disclosure forms provided by the authors are available with the full text and PDF of this article at www.ajnr.org.
References
- 1.
- 2.
- 3.
- 4.
- 5.
- 6.
- 7.
- 8.
- 9.
- 10.
- 11.
- 12.
- 13.
- 14.
- 15.
- 16.
- 17.
- 18.
- 19.
- 20.
- 21.
- 22.
- 23.
- 24.
- 25.
- 26.
- 27.
- 28.
- 29.
- 30.
- 31.
- 32.
- 33.
- 34.
- Received March 21, 2024.
- Accepted after revision July 15, 2024.
- © 2025 by American Journal of Neuroradiology