Abstract
BACKGROUND AND PURPOSE: This animal study was designed to evaluate in vivo the acute and short-term safety and efficacy of the new Artisse intrasaccular device (ISD) for aneurysm occlusion and to gain knowledge about the behavior in the aneurysms.
MATERIALS AND METHODS: The device was implanted in 7 white New Zealand rabbits with bifurcation aneurysms. Immediate and 90-day angiographic follow-up as well as histologic and scanning electron microscope imaging were evaluated.
RESULTS: Immediate postinterventional angiograms showed excellent flow reduction in all aneurysms. Progressive improvements of occlusion rate could be observed in 5 of 7 aneurysms. One device migration was noted due to undersizing, resulting in corresponding worsening of occlusion rate. Three-month microscopic examinations demonstrated excellent biocompatibility. Notably, the Artisse ISD showed increased connective tissue formation within the aneurysm sac, which correlated with the angiographic results.
CONCLUSIONS: The new Artisse ISD adapted well to aneurysm morphology and created immediate contrast stasis and excellent neck coverage. While angiographic results showed only moderate adequate occlusion at 3 months, histologic data showed excellent biocompatibility and good connective tissue formation within the aneurysm sac in all aneurysms treated with the Artisse ISD. Sizing and correct positioning appear to be crucial for adequate occlusion.
ABBREVIATIONS:
- DAPT
- dual antiplatelet therapy
- DFT
- drawn-filled tubing
- ID
- inner diameter
- IFD
- intrasaccular flow diverter
- ISD
- intrasaccular device
- mRRC
- modified Raymond-Roy classification
- NiTi
- Nitinol
- OKM
- O’Kelly-Marotta scale
- WEB
- Woven Endobridge
Various endovascular treatment modalities for cerebral aneurysms have become available over the last several decades.1 Intrasaccular flow diverters (IFDs) have expanded the armamentarium of neurointerventionalists in recent years, especially for wide-necked aneurysm at the bifurcation and along the sidewall. While several IFDs have been introduced over a decade ago, only the Woven Endobridge (WEB, Microvention) and the newer Contour (Stryker Neurovascular) are currently in clinical use.2
The newest addition to the arsenal of IFDs is the new models of the Artisse intrasaccular device (ISD), which represent an enhanced version of the formerly known device LUNA AES, and the original models of Artisse ISD (Medtronic). The key differences between the new Artisse ISD models and the first Artisse line are the shape and available size range. The new Artisse ISD is available in 1 “flared” shape of varying widths (4.5–8 mm) and heights (3–5 mm). The device is made of NiTi drawn-filled tubing (DFT) wires attached to a delivery wire that allows for electrolytic detachment and is compatible with a 0.021-inch ID microcatheter. The key differences between the LUNA AES and the former Artisse ISD devices are the shape, braid wire configuration, detachment mechanism, and compatible microcatheter. With the LUNA AES, device-related stenosis of the parent vessel was frequent, and there was a remarkable relationship between device protrusion and the occurrence of ischemic events. In contrast, the overall thromboembolic event rate remained low.3,4 Additionally, the visibility of the cage itself of the LUNA AES was poor due to the pure nitinol wire structure.
The herein discussed new device is still intended as a stand-alone therapy, without adjunctive coils placed into the aneurysm cavity.
Regarding long-term occlusion in IFDs like the WEB device, Ding et al5 proposed that it is achieved through flow stasis, subsequent thrombosis, and endothelialization. This hypothesis was supported by Rouchaud et al6 in a later study on the WEB device, in which angiographic results were correlated with the histologic occlusion results. Similar findings regarding surface endothelialization and connective tissue organization within the aneurysm sac and their correlation with angiographic outcomes have also been demonstrated with the LUNA AES.7 Additionally, histopathologic analysis was necessary to confirm the biocompatibility of these devices.
This study was designed to evaluate, in an in vivo aneurysm model, the acute and short-term safety and efficacy of the new Artisse ISD and to learn about how to use the new device correctly to achieve the best possible occlusion. This is the first study to describe the new Artisse ISD.
MATERIALS AND METHODS
The Artisse ISD
The original Artisse ISD is a self-expandable, preshaped endovascular implant made from a double layer of DFT wire mesh, made of nitinol with platinum/iridium proximal and distal marker bands available in 2 shapes. With the new Artisse there are 20 sizes ranging from 4.5–8 mm in width in a flared shape. The implants are chosen for treatment based on the aneurysm shape, width, and dome-to-neck ratio (Fig 1 and Table 1). Attached to a pusher wire that allows manipulation through a compatible 0.021 in microcatheter the device is navigated to the target aneurysm. The design allows for full retrieval and redeployment to reposition. The pusher wire is a tapered, ground core wire with platinum radiopaque markers and with a polyimide insulated cobalt chromium detachment element affixed to the distal end of the pusher wire. Detachment of the implant is achieved by using the Artisse detachment device, which delivers up to 2.1 mA of current to the detachment element via the pusher wire. The electrical current energizes the isolated detach element, causing it to dissolve, allowing separation of the implant from the pusher wire.
Photograph of the Artisse ISD. One proximal marker and one marker in the lateral part of the cage are radiopaque.
Artisse sizing charta
In Vivo Experiments
The study was performed in compliance with the Good Laboratory Practice Regulations at a federally registered test facility (Certificate Reference Number: INS-200015–0003-009).
The aneurysm model used has been previously described.8 Briefly, New Zealand rabbits under intravenously maintained anesthesia were incised medially from the mandibular angle to the manubrium sterni. After exposing the target vessels, a section of the jugular vein was removed. The left common carotid artery was ligated, cut, and guided under the pretracheal muscles to the other side. An end-to-side anastomosis was performed between both common carotid arteries, with the left artery trimmed diagonally to form a Y-shape and maintaining a small opening in the bifurcation. The previously removed vein was trimmed and sewn to the opening at the bifurcation as a blind sac. The created aneurysm was closed with a ligature. To allow for proper healing and avoid interference with the embolization process, a minimum healing period of 3 weeks was implemented.
At embolization, anesthesia was induced by subcutaneous injection of ketamine (75 mg/kg) and xylazine (4 mg/kg). Throughout the embolization, the animals were breathing spontaneously and additional anesthetic was administered via the lateral auricular vein (mixture ratio: 5:1:5; ketamine: 100 mg/mL, xylazine: 20 mg/mL, 0.9% saline: 0.08 mL/kg administered in 10-minute intervals). Acetylsalicylic acid (10 mg/kg) and clopidogrel (1 mg/kg) were administered daily 2 days prior and 30 days postimplantation. We incorporated antiplatelet medication into the protocol as a precautionary measure, because we were unable to anticipate whether dual antiplatelet therapy (DAPT) would be necessary and because DAPT was clinical practice before implantation of an IFD at the time of this study.
Antibiotic (enrofloxacin 7.5 mg/kg) and analgesic (meloxicam 0.3 mg/kg) therapy was given on the day of embolization.
A surgical cut-down of the right femoral artery was performed and a 5F Sheath (Terumo Glidesheath Slender) was inserted under an operation microscope (OPMI MD, Carl Zeiss) under sterile conditions. Heparin (500 U IV) was administered and then a 5F Codman Envoy guiding catheter was placed into the carotid artery. Using a Traxcess 0.014-inch guidewire (MicroVention) and a Rebar-18 microcatheter (Medtronic) was placed into the aneurysm cavity.
Device Sizing and Angiographic Evaluation
Biplane imaging was used for initial diagnostic runs with a 3D rotational angiogram for aneurysm sizing and finding a working projection. Aneurysm dimensions (neck width, aneurysmal height, and width) were determined and adjusted by using a reference sizing device. Due to the difficulty in determining the correct size without any prior experience in an animal model, Sim&Cure software was used to run various simulations and select the Artisse ISD size that provided the best wall apposition and complete coverage of the aneurysm sac. The device was then carefully inserted into the microcatheter and deployed at the aneurysm sac.
After deployment, the microcatheter was removed and immediate postprocedural and delayed angiographic runs were performed. Device placement and flow/stasis within the aneurysm were assessed by using standard angiographic projections. Another angiographic analysis was conducted before euthanization after 90 days. Due to the lack of a sufficient occlusion rating system for IFDs, the occlusion of aneurysms was assessed by using the modified Raymond-Roy classification (mRRC),9 which categorizes occlusion as complete (mRRC1), neck remnant (mRRC2), or residual aneurysm with contrast opacification within the coil interstices (mRRC3A) or outside the coil interstices, along the residual aneurysm wall (mRRC3B).10 Additionally, the O’Kelly-Marotta (OKM) scale was used to assign grades based on the amount of contrast filling (A, B, C, D) and the duration of contrast persistence (stasis grades 1, 2, 3) within the aneurysm lumen.11
Histopathologic Processing, Histomorphometry, and Analysis
After last angiographic control all animals were euthanized with a lethal injection of embutramid-mebezoniumiodid-tetracain solution (1 mL/10 lbs). Aneurysms and a section of the parent artery were excised, fixed on a KliniTray, and immediately fixed in 4% neutral buffered formalin. The segments of the arteries were examined for alterations of the normal structures including the regionals draining lymph nodes. Eventual nature and extent of observed reacting tissue such as hematoma, edema, encapsulation, or other gross findings were recorded. Samples were embedded in methyl methacrylate plastic after dehydration through a series of gradient alcohols and xylene. After polymerization of the methyl methacrylate, subsequent polishing with silicon carbide paper provided a coplanar even surface. The polished surface of the sample was mounted on a microscope glass slide by using optically transparent cyanoacrylate-based glue. Sections were performed at 10 µm thickness with the laser microtome (LLS ROWIAK LaserLabSolutions). The remaining block again was slightly polished and subsequently used for laser microtome sectioning. Final slides were stained with H&E stains.
The tissues were assessed by an experienced pathologist according to a 4-scale grading ranging from minimal to marked for the following parameters: platelet/fibrin thrombus, percentage of endothelialization, neointima formation on the neck surface, aneurysm sac organization, inflammation and neoangiogenesis, parent artery luminal thrombus, wall damage, overall endothelial loss, and overall inflammation (Online Supplemental Data). Additionally, scanning electron microscope imaging was performed on 2 randomly selected animals to confirm neointimal coverage at the neck of the aneurysm.
RESULTS
Angiographic Results
A total of 7 aneurysms were treated with the new Artisse model. Among these, improved contrast stasis was observed in 5 of 7 aneurysms, with an OKM scale rating ranging from B3 to C3. Notably, 1 aneurysm achieved complete occlusion after initial undersizing and choosing a large enough size thereafter (mRRC1, OKMD1). However, the remaining 4 aneurysms were still classified as mRRC2 to 3A, indicating some inflow into the aneurysm sac. At the 90-day follow-up, 1 aneurysm maintained the same occlusion rate (mRRC3A, OKMA3), while another experienced device dislocation/migration due to undersizing, resulting in neck reoccurrence (mRRC3B, OKMA3).
Importantly, none of animals exhibited device-related thrombus, vessel occlusion, or aneurysm rupture (Table 2 and Fig 2).
Angiograms of 3 aneurysms preinterventional – 1; directly after embolization – 2; and at time of control – 3. Example A shows aneurysm animal/number 4 with device migration due to undersizing and incorrect positioning. Example B shows animal/aneurysm number 7 of the Artisse with complete occlusion.
Angiographic results with occlusion according to OKM scale and mRRC
Histologic Results
Minimal to mild platelet or fibrin thrombus formation within the aneurysm was observed in all animals treated with the Artisse ISD. At the neck of the aneurysms, endothelialization and neointima formation was sufficient in all the samples. The sac organization in these aneurysms ranged from mild to moderate, with 2 animals demonstrating marked sac organization, indicating almost complete or complete occlusion of the aneurysm. In each sample, neointima formation in the aneurysm sac was observed, indicating a stabilization of the aneurysm wall. In none of the samples was a luminal thrombus or endothelial loss observed.
Overall, inflammation was only minimal and composed of giant cells and/or lymphohistiocytic reaction around the wire surfaces with occasional, moderate, lymphohistiocytic response around some of the device struts. No cell metaplasia, necrosis, or signs of local calcifications related to the implants were observed.
The process of healing of the aneurysm sac was confirmed by extracellular matrix components (Fig 3 and 4).
A, Angiography shows complete occlusion of the aneurysm at 90 days. B, The histologic image shows the coronal plane section of the bifurcation aneurysm, stained in H&E. The aneurysm sac is completely covered by organized tissue material, showing a complete conversion of the aneurysm sac consisting of stable tissue. The device is spanning over the complete neck surface. The inner aneurysm wall is intact, covered by neointima. The device demonstrates a complete occlusion of the aneurysm. C, The neck section of the bifurcation aneurysm in higher magnification (15.9×). The magnification of the selected area is pointed out in the upper right side. Marked sac organization in the neck area mainly consisting of organized tissue material. The device covering the neck is completely covered with newly formed tissue (black arrows). This stable tissue around the device occludes the neck completely and minimizes the blood flow into the aneurysm sac. The newly formed tissue covers the complete struts of the device and a newly formed endothel (black arrows) coats the inner sac lumen. Normal signs of inflammation, giant cells (red arrows) around the device struts, and the (blue) suture material are seen.
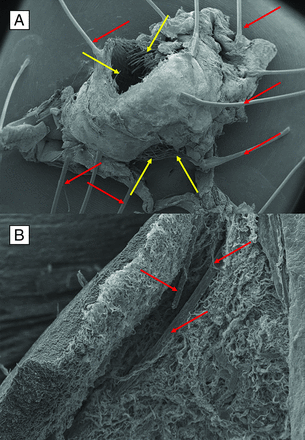
A, Scanning electron microscope (SEM) image (16×) shows an overview of the bifurcation aneurysm. Red arrows show the struts for fixing the tissue for analysis. There are no signs of perforation of the aneurysm wall or any signs of inflammation. Connective tissue covers the complete surface of the aneurysm. For SEM analysis the aneurysm is mechanically opened (yellow arrows). B, The SEM image in higher magnification (201×). The device is completely covered by newly formed tissue. The image shows the opened aneurysm including the device struts (red arrows), that are completely covered and embedded in the aneurysm wall. Fibrin and connective tissue covers the device struts without any signs of inflammation. The device is completely implemented in the newly formed aneurysm wall, indicating a good biocompatibility.
DISCUSSION
In this study, we describe the in vivo evaluation of the new Artisse ISD in an animal model. Similar to other IFDs, the Artisse ISD is designed to be fully deployed within the aneurysm sac. This design allows for both flow diversion at the neck and mechanical occlusion, potentially without the requirement of DAPT. However, at the time of the study, it was recommended to prescribe DAPT as a precautionary measure before implantation, similar to current clinical practice with other IFDs.
During deployment, it was observed that the microcatheter did not retract and that by positioning the microcatheter in the lower one-third of the aneurysm, a controlled opening was clearly visible without any catheter movement. Once the device was ready for full deployment, the microcatheter was positioned just below the aneurysm’s occlusion line to release the device with the proximal marker exactly below this line in the parent artery.
This deployment method demonstrated in our model that the device is suitable for bifurcation aneurysms, adapting well to aneurysm morphology and resulting in satisfactory immediate contrast stasis and excellent neck coverage. However, in 2 animals, the suggested Sim&Cure sizing was found to be too small. In 1 case, a larger implant was chosen, leading to an excellent position of the system—with the proximal marker in the parent artery and a good wall apposition in the whole aneurysm—resulting in complete occlusion at the 90-day follow-up.
In the other case, no larger device was available under study conditions for the given aneurysm size. The device was therefore positioned in the neck of the aneurysm leaving the dome partially uncovered by the device. Therefore, the expected device dislocation was observed during follow-up, very likely due to the inappropriate device length selection, resulting in a residual neck and a corresponding worsening in occlusion rate according to mRRC (Fig 2).
Because the device is very soft and conforms to the shape of the aneurysm, the volume of the device may be more important than the measured sizes of the dome and neck when choosing. Based on the findings of this study, including the learning curve regarding the device’s behavior, and multiple simulations with different sizes by using Sim&Cure software, we recommend oversizing the device by a minimum of 1–2 mm in both width and length of the aneurysm. A sizing chart was created based on these findings to support size selection (Table 1).
None of the Artisse ISD procedures showed acute device migration after deployment and detachment. In all cases except the aforementioned one, the location of the Artisse ISD remained unchanged. While only 57.1% of cases achieved adequate occlusion at the last follow-up according to mRRC (14.2% mRRC1, 42.9% mRRC2), it is important to note that significant contrast stasis was observed in all aneurysms. This suggests the potential for occlusion with longer follow-up.12
Upon reflection, the completely occluded aneurysm was successfully treated by using a sufficiently large Artisse ISD, which was well-positioned with the proximal marker in the parent artery. It was clearly demonstrated that an incorrect positioning of the device does not result in a stable occlusion.
Because the Artisse functions similarly to a flow diverter stent and a WEB or Contour device but its position in the parent artery is not considered in existing rating systems, the presented occlusion results do not adequately reflect the findings.
The Y-shaped structure of the Artisse at the neck, as demonstrated by immediate and follow-up angiograms, shows no vessel compromise due to the marker.
The degree of neoendotheliazation and connective tissue organization within the aneurysm sac was comparable to preclinical data of other IFDs in the Artisse ISD,5,7 and overall demonstrated a good biocompatibility.
In contrast to the LUNA AES and the Artisse ISD original line, the new model of the Artisse ISD allows aneurysms with a height of 5.4–9.6 mm and a width of 3.0–8.0 mm to be approached via a smaller microcatheter (0.021 versus 0.027 inch). Furthermore, improvements in maneuverability have been made and an electrolytical detachment system is now available. Additionally, the visibility was enhanced by using thinner DFTs. Whereas Piotin et al4 described an impact on the elastic properties and frequent shape modification with the original line of Artisse ISD in humans due to the usage of DFTs, we did not observe any significant shape modification of the current new Artisse over time. However, an extremely unusual adaption of the device to the aneurysm morphology was observed because of its soft and elastic proprieties, as soon as the correct size was chosen and available.
Based on our limited experience, we conclude that once the aneurysm is completely filled with the device, complete occlusion can be expected over time and seems to be very stable.
Limitations
The major limitation of this study is the small sample size and the short follow-up period. As it was not the intention of this study to prove stable occlusion rate over time, but to evaluate the behavior and to learn how to size and position the device in the aneurysm, no control group was used and a 3-month follow-up was sufficient to answer the study questions.
As embolization of larger aneurysms (>10 mm) was not tested in this study, the caution postulated by the authors of the preclinical study7 that the LUNA AES might not be suitable for complex, wide-necked, or fusiform aneurysms, could not be disproved with this study.
Another limitation was the DAPT, given pre- and postimplantation, which might have prevented complete occlusion of the aneurysms, although no recanalization was recorded. Further studies might answer this question.
Furthermore, not all sizes were available during the study. While Good Laboratory Practice Regulations conformity ensures objectivity, the assessment of the angiographic results were unblended.
CONCLUSIONS
The new Artisse ISD showed a very controlled deployment into the aneurysm and adapted well to the aneurysm morphology in this animal study. This resulted in immediate contrast stasis and excellent neck coverage. Correct sizing and positioning appears to be crucial for complete occlusion. A sizing chart was created based on learning during implantation and the behavior of the device. The histologic data demonstrated excellent biocompatibility, good connective tissue formation within the aneurysm sac, and neointima formation at the neck in all aneurysms, similar to other ISDs.
Long-term occlusion rates and development of the sizing chart will be shown in further studies.
Footnotes
Disclosure forms provided by the authors are available with the full text and PDF of this article at www.ajnr.org.
References
- 1.
- 2.
- 3.
- 4.
- 5.
- 6.
- 7.
- 8.
- 9.
- 10.
- 11.
- 12.
- Received March 14, 2024.
- Accepted after revision June 30, 2024.
- © 2025 by American Journal of Neuroradiology