Abstract
BACKGROUND AND PURPOSE: Lumbar disc herniation, potentially leading to nerve root compression and cauda equina syndrome, is typically evaluated using MR imaging. However, the limited availability of MR imaging outside regular hours in certain health care systems poses considerable challenges. This purpose of this study was to prospectively evaluate the diagnostic accuracy of an optimized CT lumbar spine protocol as a potential alternative to MR imaging in assessing suspected neural compression.
MATERIALS AND METHODS: Patients presenting to the emergency department with suspected cauda equina syndrome or acute radicular symptoms secondary to lumbar disc herniation referred for MR imaging were prospectively enrolled for an additional CT optimized to assess spinal stenosis. An expert radiologist, blinded to clinical data, graded canal stenosis at each lumbar level on CT. The same grading process was applied to MR imaging after a 4-week interval to maintain blinding.
RESULTS: Fifty-nine individuals were included in the final analysis. In 22 (39%) cases, no significant stenosis was identified. In a further 22 (37%) cases, disc pathology was identified that was managed conservatively. Thirteen (22%) individuals proceeded to urgent surgical decompression. In 1 (2%) instance, an alternative diagnosis was identified. Compared with MR imaging, the sensitivity, specificity, and positive and negative predictive values for CT in detecting disc pathology in patients presenting with symptoms suggestive of acute neural compression were 97% (95% CI, 82%–99%), 97% (95% CI, 83%–99%), 97% (95% CI, 92%–99%), and 97% (95% CI, 83%–99%), respectively. CT accurately identified all cases requiring urgent decompression.
CONCLUSIONS: CT accurately predicted MR imaging findings in patients with suspected cauda equina and nerve root compression, demonstrating its utility as an adjunct tool for patient triage in emergency settings with limited MR imaging access. This protocol could enhance the allocation of emergency resources by appropriately selecting patients for emergent MR imaging.
ABBREVIATIONS:
- CES
- cauda equina syndrome
- CTSS
- CT spinal stenosis
- DLP
- dose-length product
- ED
- emergency department
SUMMARY
PREVIOUS LITERATURE:
The evaluation of suspected cauda equina syndrome is dependent on MR imaging. Access to MR imaging is limited in many health care settings. CT is comparatively readily accessible and has shown some promise in the assessment of disc pathology in retrospective studies.
KEY FINDINGS:
CT was accurate in detecting cauda equina syndrome and nerve root compression secondary to disc herniation.
KNOWLEDGE ADVANCEMENT:
CT has not previously been prospectively studied for the assessment of suspected CES. Given its accuracy and relative ubiquity, CT could be a valuable tool in the assessment of these patients where MR imaging is not readily available.
Lumbar disc herniation, a common cause of back pain, often leads to localized inflammation and nerve root compression.1 Patients may present with back pain and acute radicular symptoms, including sensory disturbance and weakness, in the distribution of the affected nerve roots. The most severe outcome is cauda equina syndrome (CES), which classically manifests with saddle anesthesia, bowel and bladder disturbances, and sexual dysfunction, as well as back pain and radicular symptoms.2 Despite MR imaging being the criterion standard for the assessment of lumbar disc pathology, its limited availability, particularly out of hours, poses a significant challenge in emergency departments (EDs) globally. For instance, in the United Kingdom, round-the-clock MR imaging access is notably scarce,3 and in the United States, while 96% of EDs offer CT scans 24/7, only 66% have similar MR imaging availability.4 This disparity in access, combined with a high rate of nonsignificant findings in emergency CES referrals,5 underscores an urgent need for alternative, accessible imaging protocols. Furthermore, it would be of particular use when the overall clinical suspicion of CES may be low but cannot be safely excluded, because this could improve the selection of appropriate patients for emergent MR imaging. In addition, CT has a role in imaging emergency patients with contraindications to MR imaging such as pacemakers or spinal stimulation devices.
Our study used a prospective approach, enrolling patients presenting to the ED with symptoms indicative of lumbar disc herniation. Each patient underwent both an MR imaging and an optimized spinal stenosis CT protocol. An expert, blinded to MR imaging findings, then independently reviewed the CT imaging results. This methodology was designed to test the efficacy and accuracy of CT imaging as a viable alternative to MR imaging in emergency settings, with the aim of enhancing clinical decision-making and resource allocation in EDs experiencing limitations of MR imaging access.
MATERIALS AND METHODS
This study was approved by the Mater Misericordiae University Hospital institutional review board and ethics committee.
CT Protocol
In refining our CT protocol, we conducted preliminary scans on cadavers in collaboration with the university-affiliated anatomy lab of our hospital. A Definition AS+ machine (Siemens) was used for these examinations. The scans encompassed a range of parameters: variations in kilovolt (100–14 kV), milliampere second, field of view dimensions, reconstruction algorithms, and iterative strengths. The aim was to develop a protocol that optimally balances image quality against radiation dose, with a specific focus on enhancing the contrast between the disc and thecal sac. Lumbar disc visualization on cadaveric scans was subjectively compared with a cadaveric scan using a standard lumbar CT protocol (eg, auto-milliampere second, 100-120 kV, SAFIRE 3) as a reference The final protocol has a field of view tightly confined to the lumbar spine, using a 2-mm section thickness to provide the best signal to noise ratio. Thinner slices, for example 1-mm thickness, somewhat improved the spatial resolution but negatively affected contrast between the disc and the thecal sac.
In collaboration with the medical physics department, a protocol balancing image quality and radiation dose was selected. Scanning at 100 kV resulted in suboptimal image quality compared with higher kilovolt techniques due to relatively increased image noise. While a 140-kV scanning demonstrated superior image quality in cadaver experiments, for dose optimization and justification reasons, this setting was reserved for patients weighing over 100 kg. In these cases, the additional radiation dose was considered justified due to the potential for reduced image quality from increased adiposity. For all other patients, a 120-kV protocol was used. The use of maximum iterative reconstruction strength helped to reduce image noise compared with lower settings tested (eg, SAFIRE 3) and improve visualization of the disc relative to the adjacent thecal sac. Default windowing was optimized to increase the conspicuity of lumbar discs. The finalized optimal protocol included the following:
Auto-milliampere second with maximum-strength iterative reconstructions (eg, SAFIRE 5)
Two-millimeter reconstructions in both the axial and sagittal planes with a soft-tissue kernel, a window width of 215 and level of 40
One-millimeter reconstructions in the axial and sagittal planes with a bone kernel and standard bone window
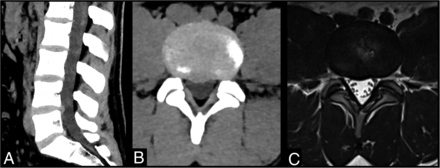
This comprehensive protocol was termed the CT spinal stenosis (CTSS) protocol (Fig 1).
An example of a high-quality spinal stenosis CT protocol for suspected CES in a 26-year-old man. A, Midline sagittal CT demonstrates relatively mild bulging discs, most notably at L4-L5 where there is a right central protrusion, but no high-grade spinal stenosis. B, Axial CT through the L4-L5 disc level in the same patient demonstrates the degree of spinal stenosis. C, Axial T2-weighted image through same L4-L5 level.
An audit comparing the dose-length product (DLP) of 20 CTSS scans with 20 routine lumbar spine CTs was performed. This audit aimed to evaluate whether our optimized protocol incurred any unexpected excess radiation dose. We selected the most recent 20 scans available for each category for this comparison.
MR Imaging Protocol
No alterations were made to the routine MR imaging lumbar spine protocol at our institution for this study, which is available on 1.5T and 3T MR imaging Magnetom Sola and Magnetom Skyra scanners (Siemens). This standard protocol encompasses sagittal T1- and T2-weighted sequences of the lumbosacral spine, extending from at least T12 to S2. It also includes axial sequences from L3 to S1. To ensure comprehensive coverage, the radiology resident reviews the sagittal images at the time of scanning and, if required, requests additional axial levels. Sequence parameters were approximately the following: Magnetom Skyra: sagittal T2WI: TR/TE = 3500/92 ms, section thickness = 4 mm; axial T2WI: TR/TE = 2870/106 ms, section thickness = 4 mm; Magnetom Sola: sagittal T2WI: TR/TE = 3500/90 ms, section thickness = 4 mm; axial T2WI: TR/TE = 4320/91 ms, section thickness = 4 mm.
Patient Cohort
Patients presenting to the ED, evaluated by emergency physicians for symptoms suggestive of cauda equina syndrome or acute radicular symptoms indicative of disc herniation, were considered for inclusion in this study. Our study specifically targeted those cases in which an urgent MR imaging referral was indicated to assess potential surgical decompression. Other potential causes of CES or nerve root compression, not typically associated with urgent surgical intervention such as epidural abscess, were not included in our study cohort. These patients who were referred for an emergency MR imaging of the lumbar spine were consecutively recruited as they presented. Patients individually consented for the research CT protocol to a radiologist while they were in the ED. The inclusion process was prospective, occurring before any imaging took place. Exclusions applied to patients with major trauma, pregnant individuals, or those who could not definitively exclude pregnancy. Additionally, patients with a history of spinal fixation were excluded because existing metalwork was anticipated to cause significant artifacts, potentially limiting the interpretability of the images.
Clinical Data
Basic demographic information and clinical examination findings from the ED assessments were meticulously recorded for each study participant. Following the initial assessment, participants underwent both the CTSS protocol and MR imaging in the ED. The eventual patient outcomes such as the final diagnosis of the presence or absence of a severe stenosis and whether conservative or surgical management of their symptoms was used were documented using data from the patient’s electronic medical record. Management decisions were determined by the treating clinical team.
Image Analysis
The CTSS protocol images were interpreted by a radiologist with 13 years of experience in emergency and musculoskeletal imaging. This expert was blinded to the patient’s details, MR imaging findings, and clinical outcomes to ensure unbiased assessment though being aware that each patient was being assessed for suspected CES secondary to disc herniation or for acute radicular symptoms.
MR imaging was designated as the reference standard for this study. The analysis involved both a qualitative assessment of the presence of cauda equina or nerve root compression and identification of the causative lesion and a quantitative measurement of the cross-sectional area of the spinal canal at each vertebral level. Central canal stenosis was graded at each disc level on the basis of the percentage of thecal sac effacement, following the criteria outlined in a study by Peacock and Timpone.6 Foraminal stenosis was graded on a scale of 0–3, as per the grading system defined by Lee et al7 and presented in Table 1. The MR images were analyzed in a similar fashion after a 4-week interval, with the radiologist remaining blinded to the patient details and the CT results to maintain consistency and avoid bias.
Grading of foraminal stenosis
Statistical Analysis
In this study, MR imaging served as the reference standard for evaluating the CTSS protocol. We calculated the sensitivity, specificity, positive predictive value, and negative predictive value, along with their binomial exact 95% confidence intervals of the CTSS in detecting disc herniation. In addition, simple descriptive statistics were used to establish the ranges for the cross-sectional area comparisons of the spinal canal. All statistical analyses were conducted using Excel (Microsoft) and the online statistics tool available at https://www.socscistatistics.com/.8
RESULTS
Patient Demographics
During an 18-month period between July 2021 and January 2023, sixty-five patients were prospectively enrolled, consisting of 26 (40%) men and 39 (60%) women. Ages ranged from 24 to 86 years, with a median age of 44 years. Of the 65 initially enrolled patients, 6 were subsequently excluded for the following reasons: 3 had emergency spinal surgeries before CT completion; 1 withdrew consent for CT after MR imaging was performed; 1 was contraindicated for MR imaging due to a spinal stimulator and was assessed exclusively with CT (which demonstrated no high-grade spinal stenosis); and 1 required contrast-enhanced 80- kV CT for suspected abdominal aortic pathology. Consequently, 59 patients were included in the final analysis.
Detailed patient characteristics, including demographics and clinical presentations, are provided in Table 2.
Patient characteristics
Imaging Findings and Subsequent Management
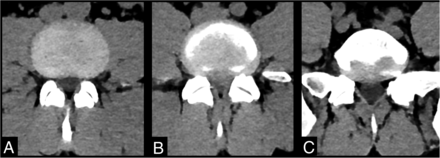
Of the 59 cases analyzed, 22 (37%) had no significant spinal stenosis. In 22 (37%), conservative management with injections and physiotherapy was chosen for the observed disc pathology. Surgical decompression was performed in 13 (22%) patients due to disc herniation (Figs 2–4). Additionally, an alternative diagnosis of an L4 wedge compression fracture, causing back pain without retropulsion or canal compromise, was identified in 1 (2%) patient. The outcome for 1 patient remains unknown because the patient was transferred back to the referring hospital post-imaging.
An example of a spinal stenosis CT protocol demonstrating to good effect a large disc extrusion in a 41-year-old woman with suspected CES. A, Midline sagittal CT demonstrates a large disc extrusion at L4-L5, which is causing a relatively high-grade spinal stenosis. B, Axial CT through the L4-L5 level demonstrates the same disc, which is central to the right subarticular zone extrusion. The degree of thecal sac stenosis can be confidently assessed, and there is severe right subarticular stenosis. C, Sagittal T2-weighted MR imaging demonstrates the L4-L5 disc herniation to a similar effect as in sagittal CT. D, Axial T2-weighted MR imaging through the L4-L5 disc level closely matches the axial CT representation of the disc herniation.
Images demonstrate the appearance of a positive case of CES using the spinal stenosis CT protocol in a 44-year-old man. A, Axial CT through the nonstenotic L3-L4 disc level. B, Axial CT through the highly stenotic L4-L5 level, which is due to a large central disc extrusion effacing the thecal sac. C, Axial CT through the L5-S1 level with normal thecal sac dimensions.
A 42-year-old female patient with suspected CES. A, Midline sagittal CT using a spinal stenosis protocol demonstrating a relatively large central disc extrusion with caudal migration at L4-L5, which is causing a high-grade spinal stenosis. B, Axial CT through the disc herniation at L4-L5. Sometimes the margins of the disc herniation are ill-defined; however, when scrolling through the axial CT, it is very evident that the thecal sac has been effaced at this level. C, Midline sagittal T2-weighted MR imaging of the same patient on the same day demonstrates the large disc extrusion with caudal migration from the L4 to L5 disc. D, Axial T2-weighted MR imaging of the L4-L5 disc level with complete effacement of thecal sac.
Performance of CTSS
Diagnostic Accuracy
All 13 cases requiring emergency surgical decompression were accurately identified on CT as having compressive lesions. The CT protocol demonstrated a sensitivity of 97% (95% CI, 82%–99%), specificity of 97% (95% CI, 83%–99%), a positive predictive value of 97% (95% CI, 82%–99%), and a negative predictive value of 97% (95% CI, 83%–99%) for detecting disc herniation, as detailed in Table 3.
2 × 2 table for diagnostic accuracy of CT spinal stenosis protocol
Level-by-Level Grading of Thecal Sac Effacement and Foraminal Stenosis
In this study, 295 central canal levels and 680 neural foramina were assessed for stenosis as per the systems used by Peacock and Timpone6 and Lee et al,7 respectively. The concordance in grading between CT and MR imaging for central canal stenosis was 99% (292 of 295 levels). Neural foramina were allocated the same grade of stenosis in 75% (515 of 690 foramina). Notably, in cases in which MR imaging identified severe foraminal stenosis (20 cases), CT corresponded with this grading in 90% (18 of 20).
Cross-Sectional Area
As an additional objective measurement to assess the concordance of CT findings with MR imaging, we compared the cross-sectional area measurements obtained from CT with those from MR imaging across the various lumbar levels where axial MR imaging cuts were available. A total of 201 lumbar levels were evaluated. We observed the following ranges of differences between CT and MR imaging measurements:
L1-L2 (n = 10): Range of difference: −0.15–0.35 cm2
L2-L3 (n = 15): Range of difference: −0.13–0.31 cm2
L3-L4 (n = 59): Range of difference: −0.25–0.3 cm2
L4-L5 (n = 59): Range of difference: −0.15–0.44 cm2
L5-S1 (n = 58): Range of difference: −0.41–0.37 cm2
These ranges reflect the variability in measurement precision between the 2 imaging modalities at each lumbar level.
Subgroup Analysis of the Cross-Sectional Area in Stenosis
We performed a subgroup analysis focusing on vertebral levels exhibiting stenosis, defined as a cross-sectional area of <1 cm.2.9,10 For the 47 levels meeting this criterion, the median values and ranges of differences between CT and MR imaging measurements are detailed in Table 4. Additionally, we assessed nonstenotic levels (cross-sectional area of >1 cm2, n = 152), with findings presented in Table 5.
Range of differences between CT and MR imaging for cross-sectional areas at stenotic levels
Range of differences between CT and MR imaging for cross-sectional area measurement at nonstenotic levels
Radiation Dose
The estimated mean DLP for CTSS scans was 579 mGy × cm, with a median of 578 mGy × cm and an interquartile range (IQR) of 549–602 mGy × cm. For routine lumbar spine CTs, the corresponding figures were a mean of 666 mGy × cm, a median of 620 mGy × cm, and an IQR of 543–690 mGy × cm. Detailed values for each scan are provided in the Online Supplemental Data.
DISCUSSION
This study prospectively evaluated the diagnostic accuracy of an optimized CT lumbar spine protocol in identifying severe lumbar disc herniation in patients suspected of having acute severe neural compression. To our knowledge, this is the first study prospectively comparing CT with MR imaging for the evaluation of suspected disc herniation. With minor modifications to a routine lumbar spine protocol, CT performed well, with high sensitivity and specificity (97% each) using MR imaging as the criterion standard.
Notably, 37% (n = 22) of the cohort demonstrated no significant stenosis, underscoring the clinical challenge of accurately diagnosing suspected CES and the pivotal role of imaging in its evaluation. In a further 37% (n = 22) of patients, conservative management was sufficient, indicating that not all cases of suspected disc pathology necessitate emergency surgical intervention even if the patient presents to the ED with acute severe symptoms. In scenarios in which CT excludes the presence of neural compression, these findings suggest that patients can be safely directed to outpatient or in-hours MR imaging for further evaluation. Conversely, when CT indicates probable compression, it can effectively guide the need for emergent MR imaging, optimizing patient triage in emergency settings when MR imaging availability is limited.
Lumbar disc herniation is a leading cause of CES, a condition necessitating urgent surgical intervention.2 The clinical diagnosis of CES is notoriously challenging, marred by its complex presentation, potential overlap with other lumbar pathologies, and confounding factors such as opioid-induced urinary retention. Despite an effort to pinpoint symptom combinations indicative of compressive lesions, clinical features alone have proved to be unreliable predictors.11⇓⇓-14 A systematic review revealed that the prevalence of CES based on positive MR imaging findings following clinical evaluation varies widely from 14% to 48%.15 This variability, coupled with the generally low level of evidence in the CES literature, which has been echoed by other authors,16 shows that clinical assessment alone is insufficient, necessitating reliable imaging for definitive diagnosis.
The limited availability of MR imaging, especially in emergency settings, compounds the challenge of managing potential CES cases. This limitation is exemplified by the dilemma of “scan-negative” patients, in whom clinical suspicion of neural compromise exists, but MR imaging fails to confirm a compressive lesion.17 Our study underscores the value of CT in this context. With its widespread availability and rapid imaging capability, CT can significantly enhance the efficiency of emergency care. For instance, a UK study reported that 24% of out-of-hours spinal center referrals required transfers for MR imaging, incurring substantial costs estimated at £6000 (US $7687.20) per transfer.18 Notably only 2.9% of patients (n = 45/1529) in this study required surgical decompression. Moreover, in a cohort of 250 neurosurgical referrals, evaluation by MR imaging was delayed until the following day for 82% of out-of-hours cases (n = 60/73).19 Delays such as these, while often unavoidable under current resource limitations, contradict recommended clinical pathways of care, which demand urgent access to imaging. For example, the national suspected cauda equina syndrome pathway published by the Getting It Right First Time Group in the United Kingdom recommends that patients presenting with suspected CES should undergo MR imaging within 4 hours.20 Given the current limitations in MR imaging availability and the ubiquity of CT, incorporating our CT protocol could improve care efficiency, especially in urgent scenarios. This integration would allow a more pragmatic approach to managing suspected cases of CES, aligning with established clinical pathways while awaiting more widespread MR imaging accessibility.
Several studies have explored the comparative effectiveness of CT and MR imaging in assessing lumbar and foraminal stenoses. Our findings align with those of Peacock and Timpone,6 who reported that CT identified CES with a sensitivity of 98%, specificity of 86%, positive predictive value of 72%, and a negative predictive value of 99%. The notably higher specificity and positive predictive value in this study may be, in part, due to differences in methodology, with the current study being prospective in nature, using a CT protocol optimized for lumbar disc assessment and the CT and MR imaging being performed during the same ED episode, compared with being obtained within 48 hours of each other as in the retrospective study by Peacock and Timpone. In addition, the smaller sample size in our study may be contributory (59 versus 151). The high sensitivity and negative predictive value observed in our study reinforce the utility of CT, not as a replacement for MR imaging but as a valuable adjunct in accurately and safely triaging patients for emergency MR imaging.
Alternative imaging modalities have also been investigated to attempt to improve the triage of individuals with suspected CES for urgent MR imaging. A prospective study of 260 cases evaluated bladder ultrasound (to assess a postvoid residual volume of > 200 mL) as an adjunct to clinical assessment for suspected CES. The authors found that the addition of bladder scanning improved diagnostic accuracy for predicting radiologic CES (sensitivity, specificity, and positive and negative predictive values of 94.1%, 66.8%, 29.9%, and 98.7%) compared with the presence of red flag symptoms such as bilateral sciatica alone.21 While ultrasound also has the benefit of relative ubiquity and is not associated with radiation exposure, CTSS overall demonstrates superior diagnostic accuracy with markedly improved specificity and positive predictive values and similar sensitivity and negative predictive values.
Despite the superior soft-tissue contrast of MR imaging, previous studies have also demonstrated the efficacy of CT in assessing canal stenosis and cord compression. Alsaleh et al22 highlighted the excellent intraobserver agreement of CT (κ = 0.96) in detecting canal stenosis. Similarly, for foraminal stenosis, Kang et al23 found that the intra- and interobserver agreement of CT paralleled that of MR imaging, especially among experienced readers.
In assessing canal stenosis, expert musculoskeletal radiologists commonly rely on qualitative assessments, as highlighted in the Delphi meeting findings.9 This approach was adopted in our study, aligning with prevalent clinical practice. Nonetheless, the challenge remains in defining an optimal measurement for spinal stenosis, given the variety of available methods.9,10 We incorporated cross-sectional area measurements of <1 cm2 as an objective adjunct to our primary qualitative analysis, in line with established quantitative criteria for spinal stenosis. This decision is supported by evidence suggesting that smaller canal cross-sectional areas correlate with more severe symptoms.24 Our analysis revealed that CT measurements were generally consistent with those of MR imaging, particularly in stenotic areas, as detailed in Tables 2 and 3. Subgroup analysis further elucidated the accuracy of CT, especially in cases with cross-sectional areas of <1 cm2, in which measurement discrepancies were smaller. This finding bolsters confidence in the reliability of CT for diagnosing canal stenosis and cord compression. Additionally, thecal sac effacement of >50% was used as a validation of the findings of Peacock and Timpone,6 concurring with the MR imaging findings in 99%. However, for foraminal stenosis, the agreement of CT with MR imaging was slightly lower at 75%, attributable to the broader grading scale used for foraminal assessment. Notably, in instances of severe foraminal stenosis (grade 3), CT findings aligned with MR imaging findings in 90% of cases, underscoring its efficacy in detecting clinically significant compressions.
Identifying the optimal approach for assessing canal stenosis remains an area of ongoing research. Recent advancements include novel techniques that use the ratio of disc-to-canal cross-sectional areas and the anterior-to-posterior diameter, offering promising results in stenosis identification and its correlation with clinical symptoms.25 Ratio-based metrics such as these that are normalized against anatomic and demographic differences may allow more robust assessment of stenosis in future studies.
A notable consideration is the radiation exposure associated with CT, which is a drawback compared with MR imaging. However, the use of our CT protocol is strategically limited to cases of suspected spinal emergencies when MR imaging is not immediately accessible, a common scenario in many regions, including ours. The mean DLP of CTSS was found to be 579 mGy × cm. This was similar to our local routine lumbar spine CT dose and is comparable with and, in some cases, lower than the CT lumbar spine DLPs reported in the literature. For example, the national dose reference level for CT of the lumbar spine in Australia for 2020 was 670 mGy × cm.26 In a more recent study from Jordan, the reported 75th percentile for lumbar spine CT was 967.7 mGy × cm,27 which is considerably higher than that in this study (602 mGy × cm). In general in the adult population, the dose associated with a CT lumbar spine is considered acceptable for the evaluation of suspected vertebral fractures. Given the potential severity of the pathology in suspected cauda equina syndrome and the resource limitations associated with emergency MR imaging, the equivalent exposure is justifiable provided the diagnostic test is accurate. Advances in dose-reduction techniques, such as photon-counting and artificial intelligence reconstruction, are anticipated to mitigate this concern, achieving comparable image quality at lower radiation doses. Our department plans to explore these innovations as they become available.
Regarding limitations, our study, while promising, had a relatively small patient sample size. Conducting larger, multicenter studies would provide more comprehensive data to validate our findings. The sample size, in terms of vertebral levels and neural foramina assessed, was substantial, yet predominantly negative for stenosis. This feature could impact the generalizability of our results, but it is noteworthy that our sample reflects the typical ED population with suspected acute nerve root compression, lending relevance to daily clinical practice. We also recognize that the use of an optimized CT protocol might create concerns about generalizability. Nevertheless, the modifications we introduced are feasible with current CT technology and can be implemented on most modern scanners. This approach, while specific to our study, adheres to principles and parameters that are achievable in various clinical settings, potentially broadening its utility in similar diagnostic contexts.
The absence of a universally accepted method for assessing and grading canal stenosis poses another challenge; to address this issue, we used multiple assessment techniques, including central canal stenosis >50%, foraminal stenosis, cross-sectional area, and qualitative assessment, enhancing the credibility of our CT evaluations. While the reader was blinded to the clinical information and outcome, the knowledge that the imaging was being performed as part of an ED assessment for acute radicular symptoms and suspected CES could introduce bias toward calling disc pathology, though the later correlation of pathology with management that demonstrated that all lesions requiring emergent decompression were correctly identified supports the radiologist’s assessment. While our study demonstrated the utility of CT in identifying cases of nerve root compression predominantly caused by disc herniation requiring urgent surgical intervention, it is important to recognize its limitations. Specifically, our study did not assess the diagnostic accuracy of CT for other causes of CES, such as intradural neoplasms, infection, and inflammation. Consequently, the generalizability of our findings to these conditions may be limited. This study does not propose CT as a substitute for MR imaging across all spinal pathologies but highlights its potential in urgent cases in which MR imaging accessibility is constrained. The inclusion of multiple raters, with varied experience levels, in future studies would enhance the robustness and generalizability of our findings. The single-reader model of this study presents a limitation in terms of variability and broader applicability.
CONCLUSIONS
Our study highlights the potential of an optimized CT lumbar spine protocol as an effective adjunct in EDs for assessing suspected CES and acute radicular symptoms suspected of being secondary to lumbar disc herniation. This study advocates the integration of an optimized CT spinal stenosis protocol into emergency radiology practice, especially where MR imaging access is limited, emphasizing its ability to effectively distinguish between cases requiring urgent intervention and those that can be managed conservatively or with delayed imaging. This approach not only has the potential to enhance patient care but also to optimize resource use in emergency health care settings.
Footnotes
Disclosure forms provided by the authors are available with the full text and PDF of this article at www.ajnr.org.
References
- 1.
- 2.
- 3.
- 4.
- 5.
- 6.
- 7.
- 8.
- 9.
- 10.
- 11.
- 12.
- 13.
- 14.
- 15.
- 16.
- 17.
- 18.
- 19.
- 20.
- 21.
- 22.
- 23.
- 24.
- 25.
- 26.
- 27.
- Received February 10, 2024.
- Accepted after revision April 11, 2024.
- © 2024 by American Journal of Neuroradiology