Abstract
BACKGROUND AND PURPOSE: During a season of high school football, adolescents with actively developing brains experience a considerable number of head impacts. Our aim was to determine whether repetitive head impacts in the absence of a clinically diagnosed concussion during a season of high school football produce changes in cognitive performance or functional connectivity of the salience network and its central hub, the dorsal anterior cingulate cortex.
MATERIALS AND METHODS: Football players were instrumented with the Head Impact Telemetry System during all practices and games, and the helmet sensor data were used to compute a risk-weighted exposure metric (RWEcp), accounting for the cumulative risk during the season. Participants underwent MRI and a cognitive battery (ImPACT) before and shortly after the football season. A control group of noncontact/limited-contact-sport athletes was formed from 2 cohorts: one from the same school and protocol and another from a separate, nearly identical study.
RESULTS: Sixty-three football players and 34 control athletes were included in the cognitive performance analysis. Preseason, the control group scored significantly higher on the ImPACT Visual Motor (P = .04) and Reaction Time composites (P = .006). These differences increased postseason (P = .003, P < .001, respectively). Additionally, the control group had significantly higher postseason scores on the Visual Memory composite (P = .001). Compared with controls, football players showed significantly less improvement in the Verbal (P = .04) and Visual Memory composites (P = .01). A significantly greater percentage of contact athletes had lower-than-expected scores on the Verbal Memory (27% versus 6%), Visual Motor (21% versus 3%), and Reaction Time composites (24% versus 6%). Among football players, a higher RWEcp was significantly associated with greater increments in ImPACT Reaction Time (P = .03) and Total Symptom Scores postseason (P = .006). Fifty-seven football players and 13 control athletes were included in the imaging analyses. Postseason, football players showed significant decreases in interhemispheric connectivity of the dorsal anterior cingulate cortex (P = .026) and within-network connectivity of the salience network (P = .018). These decreases in dorsal anterior cingulate cortex interhemispheric connectivity and within-network connectivity of the salience network were significantly correlated with deteriorating ImPACT Total Symptom (P = .03) and Verbal Memory scores (P = .04).
CONCLUSIONS: Head impact exposure during a single season of high school football is negatively associated with cognitive performance and brain network connectivity. Future studies should further characterize these short-term effects and examine their relationship with long-term sequelae.
ABBREVIATIONS:
- ACC
- anterior cingulate cortex
- BOLD
- blood oxygenation level–dependent
- dACC
- dorsal anterior cingulate cortex
- DMN
- default mode network
- HIE
- head impact exposure
- IHC
- interhemispheric connectivity
- RPFC
- rostral prefrontal cortex
- RWEcp
- risk-weighted exposure combined probability
- SN
- salience network
SUMMARY
PREVIOUS LITERATURE:
While concussions have been extensively studied, emerging evidence indicates that repetitive non-concussive head impacts significantly impact brain health in adolescent athletes, affecting brain structure as well as critical functional networks like the Default Mode Network (DMN). These impacts may also disrupt the salience network and its central hub, the dorsal anterior cingulate cortex (dACC), which are integral to cognitive performance. However, the specific effects of repetitive head impact exposure (HIE) on these networks, particularly the salience network and dACC, and their correlation with cognitive outcomes, remain underexplored, highlighting a gap in our understanding of their long-term implications.
KEY FINDINGS:
Repetitive non-concussive head impact exposure over a season of high school American football, is associated with slight cognitive impairments and alterations in brain connectivity of the dACC and salience network. Notably, affected athletes showed no improvements in verbal and visual memory tests typically seen with retesting, as found in controls.
KNOWLEDGE ADVANCEMENT:
We present novel evidence that repetitive non-concussive head impact exposure may result in connectivity disruptions of the salience network and the dACC that seems to accompany subtle cognitive impairments after a season of high school football. We also highlight that verbal memory performance may be an especially sensitive cognitive domain.
The effect of repetitive head impact exposure (HIE) on brain structure and function has been a major focus of study and debate in the field of mild traumatic brain injury, given its potential as a risk factor for adverse long-term neurologic outcomes of sport participation.1,2 Considerable research and public health resources have been devoted to studying, preventing, and properly managing concussions. However, subconcussive head impacts that are not associated with clinically diagnosed concussion constitute most HIE.3 This point is of particular concern in youth and adolescent athletes who experience concussive and subconcussive impacts at a similar rate compared with higher-level competition, given that they may be more vulnerable to deleterious effects during sensitive periods of neurodevelopment.4,5 There are noticeable associations between repetitive HIE and the connectivity of major brain networks in adolescents,6,7 especially the default mode network (DMN).8,9 Additional studies have shown that youth and adolescent athletes from multiple sports can demonstrate impairment on cognitive tests following varying degrees of repetitive HIE.10⇓-12 However, the relationship between network changes in functional connectivity and cognitive performance in the setting of subconcussive HIE remains unclear.
One of the major brain networks of concern regarding the effects of HIE is the salience network (SN), as well as its central hub, the dorsal anterior cingulate cortex (dACC). Furthermore, the SN is one of the key networks implicated in posttraumatic brain injury cognitive and psychiatric dysfunction.13 The SN is thought to coordinate the response to salient stimuli, interpreting interoceptive input and modulating visceromotor output. The SN interacts with other major networks and notably balances between the DMN and the central executive network, when transitioning between rest and task-oriented behaviors.14,15 Thus, injuries causing functional disruption within regions of the SN are of particular concern in the setting of repetitive HIE. However, the effect of repetitive HIE on the SN and dACC remains incompletely elucidated.
Recent studies have revealed associations between concussion and functional connectivity of the anterior cingulate cortex (ACC) and the SN. Collegiate athletes who sustained concussion showed increased connectivity between the ACC and other key brain regions related to executive function, which may indicate compensatory increases in functional burdens.16 Moreover, persistently decreased blood oxygen level–dependent (BOLD) functional MRI activation in the ACC has been observed during a working memory task following concussion.17 Conversely, increased functional connectivity within the SN has been correlated with preserved memory in aging, specifically on verbal memory–based tasks.18 Thus, the effect of repetitive HIE on the dACC and SN during a critical neurodevelopmental period is of particular interest, given the importance of these regions in maintaining healthy neurologic and psychiatric function.
In this study, we investigate changes in functional connectivity within the dACC and SN along with changes in cognitive performance in adolescent athletes after a single season of American high school football, examining the relationship between these changes and cumulative HIE during that season. We hypothesized that football players would demonstrate significant single-season changes in functional connectivity that would correlate with changes in cognitive scores and HIE. Additionally, we predicted that football players would demonstrate poorer cognitive performance or lack of improvement compared with control athletes.
MATERIALS AND METHODS
Overview
The imaging Telemetry And Kinematic modeLing (iTAKL) study followed high school football players throughout a football season to study the effects of repetitive HIE on the brain. The players underwent cognitive testing and MRI during the same study visit, both preseason and postseason. HIE was measured with the Head Impact Telemetry System (HITS; Simbex), which uses accelerometers embedded in the helmets. Preseason assessments were several days to 1 month before the first contact practice, and postseason assessments were performed at the earliest opportunity after the conclusion of the season (median, 26 days; interquartile range, 14–38 days). Control athletes from local sports teams were recruited for preseason and postseason cognitive testing; they also underwent pre- and postseason MRI during their study visits. Additional control athletes were recruited for cognitive testing by collaborators as part of a similar high school study conducted at the Geisel School of Medicine at Dartmouth (Hanover, New Hampshire). The control athletes participated in a variety of noncontact and limited-contact sports, including tennis, track, cross-country, rowing, swimming, golf, and baseball.
Standard Protocol Approvals, Registrations, and Participant Consents
Study protocols at each site were approved by the appropriate institutional review board at Wake Forest School of Medicine and the Geisel School of Medicine at Dartmouth. Written informed consent was obtained from a parent or legal guardian, and written assent was obtained from each study participant.
Football players 13–18 years of age at the time of enrollment were recruited from the junior varsity and varsity teams of a local high school during the 2012–2019 football seasons. Potential participants were excluded if they or their parents reported that the participant had any of the following: 1) developmental disorders, 2) neurologic disorders, 3) psychological disorders, 4) medications known to alter brain rhythm, or 5) MRI contraindications. To focus on repetitive HIE, we excluded players who experienced a clinically diagnosed concussion during the season or had reported a history of previous concussion from the analysis. Furthermore, we excluded football players who had their postseason assessments >6 weeks after the end of the season to limit the influence of factors unrelated to football participation (i.e., participation in a winter sport, deconditioning, and so forth). Board-certified neuroradiologists evaluated all MRI scans for clinically relevant abnormalities; participants with overt structural abnormalities, including but not limited to tumors, previous or acute infarcts, AVMs, or aneurysms were excluded from the study. For players with multiple years of participation in the study, we used the data from their first season for the analysis. Of 95 football players, 71 were considered for analysis (Fig 1).
Flow chart of football group exclusion steps and sample sizes for all the analyses.
A group of male high school athletes participating in noncontact and limited-contact sports, with no prior tackle football experience, were used as controls. Thirteen controls from local high school teams were enrolled in the iTAKL study and underwent the same cognitive assessments and imaging protocol as the football players at preseason and postseason time points coinciding with the pre- and postseason study visits for the football athletes. For the cognitive analysis, an additional group of 21 athletes enrolled in New Hampshire with the same pre- and postseason cognitive assessments was included to augment the size of the control group and increase statistical power. These additional control athletes were subject to the same exclusion criteria as participants in the iTAKL study and did not differ from the iTAKL controls in age or cognitive performance (Online Supplemental Data).
Cognitive Assessment
Participants took the Immediate Post-Concussion Assessment and Cognitive Test (ImPACT; https://link.springer.com/referenceworkentry/10.1007/978-0-387-79948-3_1873), a widely used computerized neuropsychological tool at their pre- and postseason study visits.19 Test administration was completed individually under the supervision of trained personnel. Data quality was reviewed and checked for validity using the Impulse Control composite scores (invalid if >30). Sixty-three of 71 football players and all 34 controls had complete and valid data for both time points (Fig 1).
Head Impact Measurement
During all practice and game sessions, football players wore helmets fitted with HITS. The HITS is a common impact measurement system in American football and uses an array of 6 single-axis accelerometers that are spring-loaded to maintain contact with the head.20⇓-22 To avoid recording spurious impacts, we set the lower limit of data acquisition to a peak linear head acceleration of 10 g. Additionally, videographic recording and review of each session was conducted to ensure that all impacts not directly related to helmeted athletic participation were removed from the data set. The composite metric risk-weighted exposure combined probability (RWEcp) was used to capture cumulative HIE, as detailed in prior studies.23⇓-25 Of the 63 football players with complete cognitive data, 58 had HITS data sufficient to compute the HIE metrics; of the 57 football players with complete imaging data, 51 had HITS data sufficient to compute HIE metrics. A total of 50 football players had complete cognitive data, imaging data, and HIE metrics (Fig 1).
MRI Acquisition
All football players were scanned during their pre- and postseason assessments; 13 iTAKL controls were scanned at similar time points, which, as mentioned above, were timed to coincide with the football players’ study visits and did not always coincide with the start and end of their respective sport season. All MRI sequences throughout the study were acquired on the same 3T Magnetom Skyra MRI scanner (Siemens) using a 32-channel head/neck radiofrequency coil. Participants were supine and were instructed to remain still with their eyes open during acquisition. The resting-state BOLD data were acquired for 6.3 minutes using a gradient-echo-planar imaging sequence with the following parameters: TR = 2000 ms, repetitions = 190, TE = 25 ms, flip angle = 90°, field of view = 224 × 224 mm, 3.5-mm3 isotropic resolution. Field map imaging was performed using a double-echo gradient-echo sequence (TR = 488.0 ms, TE = 4.92/7.38 ms, voxel size = 3.5 × 3.5 × 3.5, flip angle = 60°) that generated 2 magnitude images and 1 phase image. Structural T1-weighted images were acquired for spatial normalization using a 3D MPRAGE sequence with the following parameters: TR = 2300 ms, TE = 2.98 ms, flip angle = 90°, FOV = 256 × 256 mm, 1-mm3 isotropic resolution.
Imaging Data Preprocessing
Field-map distortion correction of the BOLD data was performed using the standard tool FUGUE in FSL (https://fsl.fmrib.ox.ac.uk/fsl/fslwiki/FUGUE). All further preprocessing was performed with Statistical Parametric Mapping 12 (SPM12) software (http://www.fil.ion.ucl.ac.uk/spm/) as implemented in the CONN functional connectivity toolbox (Version 20.b; https://web.conn-toolbox.org/) preprocessing pipelines,26 running on Matlab R2015b (MathWorks). Imaging data were processed using a standard CONN preprocessing pipeline. T1-weighted images were realigned and normalized to the Montreal Neurological Institute template and used to generate tissue segmentations of gray matter, white matter, and CSF. Resting-state BOLD data-processing included realignment, slice-timing correction, direct segmentation and normalization into Montreal Neurological Institute space, and spatial smoothing with an 8-mm3 full width at half maximum Gaussian kernel. For motion artifact detection and removal, volumes or scans with the following cutoffs were flagged as outliers: 1) scan-to-scan global BOLD signal change of >3 SDs, or 2) >1-mm frame-wise displacement. The denoising step included scrubbing of the flagged outlier scans, regression of the 6 motion parameters and their first-order derivatives, and the removal of white matter and CSF-related noise on the basis of the first 5 principal components estimated from the BOLD signal of these segments using the anatomical component correction (aCompCor) process, which is a component-based noise-correction method.27,28 After scrubbing and nuisance regression, images were detrended and bandpass-filtered between 0.008 and 0.1 Hz. After denoising, 2 football players with >20% of outlier volumes per scanning session (>38/190 volumes) were excluded from the imaging analysis due to their excessive motion; an additional 12 football players were excluded during a visual quality check of the BOLD data, principally for incomplete brain coverage.
Functional Connectivity Measures
Fifty-seven football players and the 13 controls were included in the functional connectivity analysis, performed using the CONN functional connectivity toolbox (Version 20.b).26 Interhemispheric connectivity (IHC) at each voxel was computed by calculating the Fisher-transformed correlation coefficient between the BOLD signal at a given voxel and that of the corresponding voxel in the contralateral hemisphere. To calculate IHC for our ROI, the dorsal ACC, we averaged the IHC of all voxels in the right dACC (Brodmann area 32) as delineated by the Brodmann atlas available within CONN. Within-network connectivity of the SN was calculated by averaging the Fisher-transformed pair-wise correlations for all the ROI pairs in an ROI-to-ROI connectivity matrix of SN regions, defined in the standard networks atlas in CONN.29 These SN ROIs were obtained from an independent component analysis of 497 subjects from the Human Connectome Project (https://www.humanconnectome.org/) and included 7 regions: the joint anterior cingulate cortices, right and left anterior insulae, right and left supramarginal gyri, and right and left rostral prefrontal cortices (RPFC). Connectivity measures were exported from CONN for further statistical analysis.
Statistical Analyses
Cognitive Data.
We first compared demographic information and test-retest intervals between football players and controls. We also examined their ImPACT performances for distributional attributes and outliers. Baseline (preseason) and postseason ImPACT scores for the 2 groups were compared using a linear mixed model implemented in jamovi (https://www.jamovi.org/) using the GAMLj module (https://gamlj.github.io/), which integrates the R-based package lme4 (https://cran.r-project.org/web/packages/lme4/index.html).30,31 Fixed effects included time point, group, and time point by group interactions to compare the change from baseline of the 2 groups. We also included the test-retest interval as a fixed-effect covariate to control for the variability in practice effects. Participant identifiers were the random effects in the model.
ImPACT scores were also analyzed using a regression-based z score approach.32 This method is like a reliable change index and enables the identification of participants who underperformed during their postseason assessment. By means of multiple regression models incorporating baseline score, age, and test-retest interval, we calculated a predicted range of postseason scores for each metric based on the control athletes’ data. For each participant, z scores for each metric were computed for both groups and represented the difference between the actual postseason score and the predicted postseason score. Before data analysis, a z score cutoff of >1.5 SD lower than the predicted value was selected to identify poor performers. We used χ2 tests to determine whether the football group contained a disproportionate number of poor performers for each metric.
Functional Connectivity
Due to the disproportionate sample sizes, with only 13 control athletes versus 57 football players, we refrained from comparing connectivity-change differences between the groups, because the limited control data may lead to unreliable inferences. Preseason-to-postseason changes in the IHC of the dACC and SN connectivity were assessed independently in the football and control groups using paired-sample t-tests in jamovi. Additionally, connectivity measures of the football group were examined for correlations with ImPACT Δ scores (post- minus preseason). Due to the small sample size of the control group, analyses are underpowered but were performed to reveal the direction of change. To examine which subregions of the SN drove connectivity changes, we performed ROI-to-ROI connectivity analysis within the SN for each group independently in CONN to visually illustrate significant changes at the connection level (P value uncorrected, < .05).
Correlations with RWEcp
For the football players, Δ scores for ImPACT composites, IHC of the dACC, and average within-network connectivity for the SN were calculated. The Pearson or Spearman correlation was used to evaluate the linear correlation between these change measures and RWEcp.
RESULTS
Participants
The football and control athletes included in the cognitive analyses did not differ significantly in age (football group: 16.4 [SD, 1.2] years versus controls: 16.5 [SD, 1.2] years), but the test-retest interval was longer for football players than for controls (football group: 136.9 [SD, 23.3] days versus controls: 116.5 [SD, 31.7 ]days, P < .001). The football and control athletes included in the connectivity analyses did not differ significantly in age (football group: 16.5 [SD, 1.1] years versus controls: 16.8 [SD, 1.1] years) or in the scan test-retest interval (football group: 139.9 [SD, 20.3] days versus controls: 137.7 [SD, 28.6] days).
Cognitive Performance
ImPACT Scores.
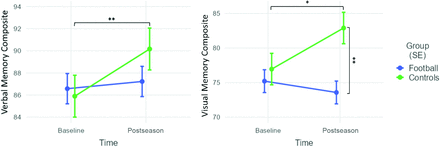
Table 1 shows the pre- and postseason results of the ImPACT scores for the football players and the control athletes. Preseason performance was similar, though the control group performed better than the football group on the Visual Motor composite (P = .04) and the Reaction Time composite (P = .006). For postseason performance, the control athletes group scored significantly higher than football players on the Visual Memory composite, Visual Motor composite, and the Reaction Time composite (P = .001, P = .003, and P < .001, respectively). There were also significant group-by-time point interactions for the Verbal Memory composite (P = .04) and the Visual Memory composite (P = .01). Figure 2 shows how the control group had significantly greater improvement from baseline to postseason on these composites. Also, the local iTAKL controls and the controls from New Hampshire showed no differences in pre- or postseason ImPACT scores (Online Supplemental Data).
Changes in cognitive performance in ImPACT composites that had a significant time point by group interaction. Football players (n = 63), control athletes (n = 34). Single asterisk indicates P < .05; double asterisks, P < .01. SE indicates standard error (Whiskers represent the standard error).
Baseline and post-ImPACT composite raw scores in football players versus control athletes
Regression-Based z-Score Analysis
On the basis of the z scores from the regression models that accounted for baseline scores, age, and test-retest intervals, the football group had a significantly higher percentage of players who performed ≥1.5 SDs below their predicted level on the Verbal Memory composite, Visual Motor composite, and the Reaction Time composite compared with the control group (Table 2).
Regression-based z scores for postseason ImPACT composite scores for football players (n = 63) and control athletes (n = 34)a
Functional Connectivity Measures
The football group had significant decreases postseason in both dACC IHC and the average within-SN connectivity. There were no significant changes for the control group, but a nonsignificant drop in within-SN connectivity was observed (Fig 3). Within-SN ROI-to-ROI connectivity suggested that different patterns of connectivity decrease at the connection level might drive overall within-SN connectivity in each group. In football players, ACC to the right RPFC (P = .01), ACC to the left RPFC (P = .01), and ACC to the anterior insula (P = .02) exhibited significant decreases during the test-retest interval. In controls, the ACC to the right RPFC (P = .01) and right RPFC to the left RPFC (P = .03) exhibited significant decreases during the test-retest interval (Online Supplemental Data).
Changes in connectivity in football players (n = 57) and control athletes (n = 13). SE = standard error (Whiskers represent the standard error)
In the football players, decreases in the dACC IHC significantly correlated with increases in the ImPACT total symptom score (P value = .03, Spearman ρ = −0.3). Additionally, decreases in within-SN connectivity correlated significantly with decreases in the Verbal Memory composite (P value =.04, r = 0.29; Table 3).
Relationship between change in ImPACT scores and change in connectivity measures in football players n = 50
RWEcp
Increased HIE as measured by RWEcp was associated with significant slowing in reaction time (P value = .03, Spearman ρ = 0.29), and significant increases in total symptom scores (P value = .006, Spearman ρ = 0.36). There were no significant correlations between RWEcp and dACC IHC or within-SN connectivity (Table 4).
Relationship between head impact exposure and change in connectivity and cognitive measures
DISCUSSION
Our findings demonstrate that repetitive head impacts during a season of American football without a clinical diagnosis of a concussion are associated with subtle cognitive decrements and connectivity changes in the adolescent brain. Football players showed no improvement in their ImPACT Verbal Memory and Visual Memory composite scores, while controls showed significant improvement in both composites. In addition, there was a significantly higher proportion of these contact athletes who performed >1.5 SDs below predicted scores on the ImPACT Verbal Memory, and Visual Motor and Reaction Time composites, corroborating a similar finding in a cohort of collegiate contact sport athletes.12 At postseason, football players had decreased dACC IHC and within-SN connectivity, correlating with poorer total symptom scores and verbal memory performance, respectively. The control group also exhibited a decrease in within-SN connectivity, albeit not statistically significant, and the specific connections that drove this decrease in within-SN connectivity were different in the football players. This finding may reflect developmental pruning, and future studies are needed to explore SN development in adolescents and adolescent athletes.
These findings are consistent with previous literature and further strengthen the case for a relationship between repetitive HIE and neurocognitive performance.10⇓-12,33 Despite the widespread use of ImPACT, student athletes with a high-impact burden do not regularly undergo repeat ImPACT administration or clinical evaluation in the absence of a diagnosed/suspected concussion. These findings highlight the importance of studying athletes who fail to demonstrate the expected improvement between pre- and postseason assessments and suggest that more diligent monitoring of symptoms and cognitive functioning may be a reasonable precaution to protect adolescent development.
These changes in connectivity build on previously reported connectivity abnormalities in critical brain regions and networks following a concussion in youth and adolescent athletes.8,9 The right and left dACC are hubs for the SN connected by tracts within the anterior corpus callosum that may be susceptible to repetitive HIE.34 While structural abnormalities in the corpus callosum are reported in collision sport athletes after concussion,35⇓-37 functional measures such as dACC IHC may provide a more sensitive measure of the integrity of the corpus callosum that precedes or predicts the risk of changes in metrics derived from DTI. This possibility is especially relevant in younger athletes undergoing maturation of important white matter tracts.
Previous studies have shown that RWEcp or similar measures had strong correlations to changes in fractional anisotropy, mean diffusivity, and other diffusion measures of microstructural integrity.10,11,33 If functional connectivity changes are mediated primarily by changes in white matter, then it would be reasonable to expect a relationship between RWEcp and functional connectivity. We did not find any correlations between RWEcp and changes in dACC IHC or within-SN connectivity. We found modest-but-significant correlations between RWEcp and changes in the Reaction Time composite and Total Symptom Score. These findings may reflect the limitations of the current analysis, especially regarding statistical power, but they may also suggest a multifactorial relationship among HIE, microstructural damage, and compensatory/pathologic functional changes.
There are some limitations in interpreting these results. This cohort was limited to male high school football athletes, so the generalizability of these findings to female athletes or other sports with different HIE profiles remains in question. Few controls have been enrolled in the iTAKL study, limiting statistical power and hindering direct between-group statistical comparison of imaging-based measures. The football players were not assessed during the season or at an equally immediate time following the season, and there are no data to extrapolate the HIE burden (i.e., from a winter sport) or degree of HIE cessation after the end of the season. This issue made it difficult to disambiguate the immediate effect of the football season from an individual’s capacity to recover and may reduce the magnitude of between-group differences in the cognitive data and connectivity measures. Last, given the lack of statistical power in the control group noted above and the exploratory nature of the analysis, we did not adjust for multiple comparisons. Replication in a properly powered cohort will be crucial to confirm and further elaborate on these findings.
CONCLUSIONS
The current findings offer a framework and preliminary evidence regarding the relationship among repetitive HIE, connectivity of the SN, and cognitive performance. There is no doubt that subject and environmental factors, such as HIE, interact to confer unique effects. Future studies that more precisely account for interactions between subject-level factors and HIE are needed to accurately address the public health concerns posed by head impacts in youth and adolescent contact sports.
Acknowledgments
We thank all participants and their parents or guardians for their commitment to this research.
Footnotes
This work was supported by grants from the National Institutes of Health (grant Nos. R01 NS082453, R01 NS091602, R01 CE001254).
Disclosure forms provided by the authors are available with the full text and PDF of this article at www.ajnr.org.
References
- 1.
- 2.
- 3.
- 4.
- 5.
- 6.
- 7.
- 8.
- 9.
- 10.
- 11.
- 12.
- 13.
- 14.
- 15.
- 16.
- 17.
- 18.
- 19.
- 20.
- 21.
- 22.
- 23.
- 24.
- 25.
- 26.
- 27.
- 28.
- 29.
- 30.
- 31.
- 32.
- 33.
- 34.
- 35.
- 36.
- 37.
- Received January 20, 2024.
- Accepted after revision March 18, 2024.
- © 2024 by American Journal of Neuroradiology