Graphical Abstract
Abstract
BACKGROUND AND PURPOSE: Primary trigeminal neuralgia (PTN) is a prevalent chronic pain disorder. This condition is believed to be associated with demyelination of the trigeminal nerve. Previous studies in this field have focused on diffusion tensor imaging, which has limited sensitivity and specificity to myelin. In the present study, we assessed the trigeminal nerve root via the macromolecular proton fraction (MPF) mapping technique. MPF demonstrated strong correlations with myelin histology in a number of earlier animal studies and is currently viewed as a promising clinical myelin biomarker.
MATERIALS AND METHODS: We performed a prospective case-control study. Fifty-six patients with unilateral PTN and 27 healthy controls were included. All participants were evaluated by using high-resolution brain MR imaging, which included the MPF technique. MPF values from different parts of the trigeminal nerve root, such as the root entry zone (REZ) and central and lateral cisternal segments, were extracted. ANCOVAs were performed. Correlations between MPF values and Sindou grade, duration, and intensity of symptoms were also evaluated in patients with PTN.
RESULTS: A statistically significant decrease in the average MPF of the affected trigeminal nerve root was observed in the PTN group compared with the healthy control group (P < .01, false discovery rate [FDR] corrected). Specifically, reductions in the MPF values of the REZ and central cisternal parts of the affected trigeminal nerve root were found in patients with PTN (P < .01 and P < .05, respectively, FDR corrected). Furthermore, we identified a decrease in the average and REZ MPF values on the affected side compared with the contralateral side in patients with PTN (P < .05 and P < .001, respectively, FDR corrected). A negative correlation between MPF values in the REZ and Sindou grade was revealed (R = −0.35, adjusted P < .05).
CONCLUSIONS: Our preliminary results suggest that MPF could serve as a new neuroimaging biomarker of trigeminal nerve root impairment in patients with PTN and enable noninvasive detection of nerve root demyelination.
ABBREVIATIONS:
- BPI
- Brief Pain Inventory
- FA
- flip angle
- FDR
- false discovery rate
- IQR
- interquartile range
- MD
- mean between-group differences
- MPF
- macromolecular proton fraction
- MT
- magnetization transfer
- PD
- proton attenuation
- PTN
- primary trigeminal neuralgia
- REZ
- root entry zone
- TN
- trigeminal neuralgia
SUMMARY
PREVIOUS LITERATURE:
The exact pathogenesis of PTN remains unclear, but histological studies indicate a notable level of myelin damage associated with the condition. Earlier neuroimaging research primarily employed DTI to evaluate microstructural damage in the trigeminal nerve of PTN patients. However, no myelin-specific techniques have been used to assess myelin damage in the human trigeminal nerve to date. Recent literature suggests that fast MPF mapping is highly specific for myelin. This study is the first effort to apply fast MPF mapping to PTN imaging.
KEY FINDINGS:
We observed a statistically significant reduction in MPF values in the affected trigeminal nerve root of the PTN group compared to the healthy controls, indicating myelin impairment. The decrease in myelin within the REZ segment was correlated with the severity of neurovascular compression, underscoring the clinical importance of myelin damage.
KNOWLEDGE ADVANCEMENT:
This study highlights myelin-related alterations in the trigeminal nerve root of PTN patients and suggests the potential of fast MPF mapping for future clinical research.
Primary trigeminal neuralgia (PTN) is a prevalent chronic facial pain condition1 characterized by sudden and intense pain attacks along the branches of the trigeminal nerve. The incidence rate of this condition reaches 0.3%, with middle-aged women believed to be the most affected.1 The main cause of PTN is neurovascular conflict (classic trigeminal neuralgia [TN]), but in some cases, there are no documented structural alterations in the trigeminal system (idiopathic TN). When conventional pain management methods are ineffective, surgical intervention is considered a major option for treating patients with PTN.1 However, even after microvascular decompression, there are patients who do not show improvement or experience a decline in their clinical status, necessitating further treatment options, particularly in cases of idiopathic TN.2
The pathogenesis of PTN is not fully understood.3,4 Histologic examinations have shown that compression of the trigeminal nerve is associated with a notable degree of myelin impairment from inflammation, particularly at the root entry zone (REZ).5⇓-7 This results in pathologic demyelination and remyelination of the affected nerve.8,9 In addition, there is evidence of axonal dystrophy and Schwann cell damage associated with nerve root compression.4 For idiopathic TN, proposed pathologies include neuronal voltage-gated ion channel dysfunction, neuroinflammation, and nonspecific lesions in the brainstem.3 It is also thought that the sensitization of the trigeminal pathway, along with the pain-modulating circuits in the brain, may play a crucial role in the pathophysiology of the disease.4
MR imaging of the brain is a crucial diagnostic tool for individuals with TN.10 It offers comprehensive insights into the anatomy of the posterior fossa and enables the identification of the underlying cause of TN, facilitating clinical decision-making and treatment algorithms.11⇓-13 It is essential for the diagnosis of secondary TN, which occurs when pain is related to an existing neurologic condition, such as multiple sclerosis or a tumor.3 Sophisticated neuroimaging techniques, such as DTI, allow for quantitative analysis of microstructural alterations in the nerve root.14 Several studies utilized these methods in patients with PTN to assess trigeminal nerve microstructural impairment or to evaluate surgical decompression outcomes.15⇓⇓-18 Despite their numerous benefits, diffusion metrics have restricted ability to detect and evaluate demyelination accurately due to their limited sensitivity and specificity to myelin.19 However, accumulating evidence suggests that myelin dysfunction is a critical component of PTN development.3,4
The application of specialized myelin imaging methods20,21 could provide a promising approach to assess trigeminal nerve root microstructural damage in patients with PTN. To the best of our knowledge, none of such methods were used to assess myelin damage in the trigeminal nerve in humans to date. One of the quantitative myelin imaging techniques, fast macromolecular proton fraction (MPF) mapping,22,23 is based on the magnetization transfer (MT) effect and measures a relative amount of immobile macromolecular protons involved into magnetization exchange with mobile water protons. The MPF mapping method combines source data obtained from gradient-echo images with MT, T1, and proton attenuation (PD) contrast weightings, to reconstruct MPF maps by using the single-point algorithm.24 MPF has been validated as a reliable myelin biomarker in various animal model studies, showing a strong linear correlation with the myelin content in both gray and white matter.25⇓⇓-28 Moreover, this method has shown potential as a targeted clinical tool to evaluate myelin damage in different pathologic conditions.29⇓-31 MPF has a high specificity for myelin and offers superior measurement accuracy compared with other myelin imaging biomarkers.32 It has been shown that MPF mapping provides high reproducibility,33 independence of magnetic field strength,34 and insensitivity to iron deposition,30 axonal architecture,28,35 or alterations in nonmyelin cellular elements.35 In the synthetic-reference variant22 complemented with data-driven B1 nonuniformity correction,24 MPF maps can be calculated from 3 source images, resulting in a clinically feasible acquisition time (less than 5 minutes for 3T MRI systems). Furthermore, fast MPF mapping enables generation of high-resolution images (with voxel size on the order of 1 mm3), allowing visualization of even tiny anatomic structures such as cranial nerves. Given these technical benefits, it would be logical to explore the potential of MPF mapping to assess trigeminal nerve root in individuals with PTN.
To date, there is a lack of studies examining trigeminal nerve root through MPF mapping or other targeted myelin imaging techniques. Therefore, our objective was to assess the differences in MPF values within the cisternal part of the trigeminal nerve root between individuals with PTN and controls. Our hypothesis was that patients with PTN would exhibit microstructural changes in the nerve root due to demyelination, resulting in reduced MPF values. Additionally, we assumed that variations in MPF values would be associated with both the degree of nerve root compression and the severity of the disease.
MATERIALS AND METHODS
Participants
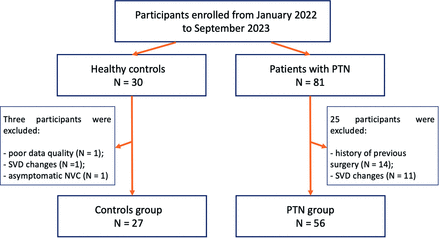
The subjects of this prospective case-control study were patients who were diagnosed with PTN and surgically treated at our hospital between January 2022 and September 2023 and healthy controls of the same age and sex. All participants underwent high-resolution brain MRI and detailed neurologic assessment before surgery. Initially, 111 participants, including 81 patients with PTN and 30 controls, were enrolled. The flow chart for the selection of patients is shown in Fig 1. The inclusion criteria for patients with PTN were as follows: 1) a diagnosis of PTN based on the International Classification of Headache Disease, Third edition36; 2) 18–80 years of age; and 3) acceptable-quality MRI data. The exclusion criteria for both groups were as follows: 1) comorbidity with other pain disorders; 2) secondary cause of TN; 3) other neurologic, psychiatric, cardiovascular, or oncologic comorbidities; 4) MRI signs of small vessel disease (Fazekas score > 1); 5) a history of previous neurosurgical interventions; and 6) poor MRI data quality. Data were considered of poor quality if either the bilateral trigeminal nerves could not be identified on MPF maps or there were visible motion artifacts overlapping with the REZ or the cisternal part of the nerve. Each patient signed a written informed consent form to participate in the study. The study was carried out according to the Declaration of Helsinki and was approved by the local Ethics Committee of the Federal Center for Neurosurgery, Novosibirsk, Russia (protocol no. 7 dated 05-25-2021). Our study followed the Strengthening the Reporting of Observational Studies in Epidemiology methodology37 and the checklist is provided in the Supplemental Data.
Flow chart of the participant inclusion process. NVC = neurovascular conflict; SVD = small vessel disease (MRI changes consistent with Fazekas score 2 or 3).
Pain Intensity Assessment
To estimate the severity of patient pain sensations, the Brief Pain Inventory (BPI) was used.38 It is a method based on a 11-point numerical rating scale (0–10), asking the patient to rank their minimal, maximal, and average pain intensity for the past week. We used mean BPI values for the following correlation analysis.
MRI Data Acquisition
MR imaging data were acquired by using a 3T system (Ingenia, Philips Healthcare) equipped with a 16-channel receiver head and neck coil. The MRI protocol included conventional MRI sequences (details provided in the Supplemental Data), as well as the fast MPF mapping protocol. The method relies on reconstructing MPF maps by iteratively solving the pulsed MT equation at the voxel level by using 3 source images with MT, T1, and PD contrast weightings,23 along with a synthetic reference image for data normalization22 and data-driven surrogate B1 field calculation for B1 inhomogeneity correction.24 The whole-brain MPF mapping protocols included 3 spoiled gradient-echo sequences with the following acquisition parameters:
MT-weighted: TR = 50 ms, TE = 4.60 ms, flip angle (FA) = 10°, scan time 2 minutes 21 seconds;
T1-weighted: TR = 20 ms, TE = 4.60 ms, FA = 20°, scan time 0 minutes 56 seconds;
PD-weighted: TR = 20 ms, TE = 4.60 ms, FA = 4°, scan time 0 minutes 56 seconds.
For off-resonance saturation in the MT-weighted sequence, the standard manufacturer’s Gaussian-filtered 3-lobe sinc pulse with the duration of 23.5 ms, offset frequency of 1100 Hz, and effective FA of 520° was applied. Scan acceleration was achieved by the combined use of multishot echo-planar readout (EPI factor 5) and parallel imaging SENSE with acceleration factors 1.2 and 1.9 in the phase and slice directions, respectively. All images were obtained in the sagittal plane with a nominal voxel size of 0.625 × 0.625 × 0.7 mm3 after interpolation (matrix 384 × 384 × 271, field-of-view 240 × 240 × 190 mm3), with no partial Fourier transform. The actual voxel size was 1.4 × 1.4 × 1.4 mm3. Example source images obtained with the above protocol are shown in the Supplemental Data.
Image Processing and Analysis
MPF maps were reconstructed by using custom C++ language software (available at https://www.macromolecularmri.org/) based on the single-point synthetic reference algorithm with surrogate B1 field correction, detailed elsewhere.24 After the MPF calculations, the images were converted from Analyze to DICOM format by using wfu_dicomtk (SPM5-based toolbox, https://www.nitrc.org/docman/view.php/195/523/WFU_Pipeline.pdf). Further analysis of MPF maps was performed via Horos DICOM Viewer software (https://horosproject.org) by using manually created oblique multiplanar reconstructions along the TN root axis. Circular ROIs were set in the oblique axial plane at the level of the REZ, as well as in the central and lateral portions of the cisternal part of the trigeminal nerve root. The area of the ROI varied from 1.2 mm2 to 2.5 mm2. Additionally, we averaged the regional values from 3 ROIs and calculated the mean MPF value from the cisternal part of the trigeminal nerve root. Measurements were performed by 2 certified radiologists (with 3 years and 7 years of experience), who were blinded to the information about the patient group, and the averaged values were included in subsequent analysis. A representation of the ROIs is shown in Fig 2. Additionally, cerebellopontine angle structures were visually evaluated with 3D T2 fast field echo and 3D TOF sequences for each patient to assess the presence and severity of neurovascular conflict, according to the Sindou classification39 (detailed in the Supplemental Data). Fig 3 shows a representation of MPF maps in patients with different grades of compression. To assess TN root atrophy, we measured root thickness by using 3D T2 fast field echo in the oblique axial plane at the level of REZ (2 mm laterally from the pons).
Illustration of the ROIs positioned on MPF maps. A, The cisternal portion of the trigeminal nerve root in an oblique axial view; B, The ROIs measured 1.6 mm2 in size at the root entry zone (marked by a red circle), central segment (marked by a blue circle), and lateral segment (marked by a green circle).
Examples of MPF maps of patients with grade 1 (A), grade 2 (B), and grade 3 (C) neurovascular conflict. Oblique axial slices along the cisternal part of the TN root.
Statistical Analysis
Statistical analysis was performed by using R software (www.r-project.org). Our data were normally distributed according to the Shapiro-Wilk test. Interrater agreement was assessed by using the intraclass correlation coefficient based on the absolute agreement paradigm with the 1-way random effects model by using the ‘psy’ library in R as detailed in the Supplemental Data. A 1-way ANCOVA was conducted to compare the MPF values of various segments of the cisternal part of the trigeminal nerve root among different groups. The analysis included comparisons between the affected and contralateral sides in individuals with PTN, the affected side in individuals with PTN and healthy controls, and the contralateral side in individuals with PTN and healthy controls, while controlling for age and sex as covariates. We provide the mean between-group differences (MD) along with their 95% CI values in the main text of the article. F-values and corresponding exact P values are reported in the Supplemental Data. Correlation analyses (Spearman correlation tests and Spearman partial correlation tests controlling for the effects of age) were performed among the MPF values, Sindou grade, pain intensity, and disease duration. Pearson correlation and Pearson partial correlation analyses between MPF values and TN root thickness were also performed. A P value less than .05 was regarded as statistically significant (after FDR correction with the Benjamini-Hochberg procedure).
RESULTS
Clinical and Demographic Data
The clinical and demographic information of the participants enrolled is presented in the Table. Fifty-six patients with PTN (22 men and 34 women, 30–75 years of age, average 57.6 ± 10.9 years) and 27 healthy controls (13 men and 14 women, 41–75 years of age, average 59.1 ± 7.5 years) ultimately participated in the study (Fig 1). The PTN and healthy control groups did not differ in age (P > .1) or sex (P > .1). In the PTN group, 35 patients had pain on the right side, and 21 patients had pain on the left side. Neurovascular compression was identified in 43 patients (with 17 classified as grade 1, 10 as grade 2, and 16 as grade 3 according to Sindou). In 13 patients, no neurovascular compression or other structural abnormalities were found in the trigeminal system, indicating idiopathic TN. The median duration of the disease was 7 (interquartile range [IQR] = 7) years, and the median average pain intensity was 5 (IQR = 2) points according to the BPI.
Participants’ characteristics
Comparison of the Regional MPF Values in the Right versus Left Trigeminal Nerve Root in Healthy Control Group
There were no significant differences in MPF values between the cisternal parts of the right and left trigeminal nerve roots in the healthy control group as per ANCOVA (P > .1 for all segments of the cisternal part of nerve root, Supplemental Data); consequently, these data were combined into a single group for further analysis to increase statistical robustness.
Comparison of the Regional MPF Values between Patients and Controls
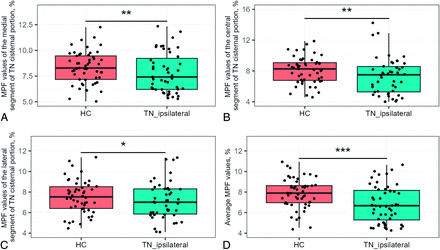
The average MPF value was significantly lower in the affected nerve root of patients with PTN than in those of controls (MD = −1.15, 95% CI [−1.73 to −0.56], P < .001, FDR-corrected, Fig 4D). Specifically, we observed a reduction in MPF in the REZ region (MD = −1.12, 95% CI [−1.86 to −0.38], adjusted P < .01, Fig 4A), in the central (MD = −1.42, 95% CI [−2.25 to −0.59], adjusted P < .01, Fig 4B) and in the lateral (MD = −0.84, 95% CI [−1.58 to −0.09], adjusted P < .05, Fig 4C) segments of the cisternal part of the affected trigeminal nerve (Supplemental Data). Additionally, a decrease in MPF values was detected in the central segment of the contralateral nerve root of patients with PTN compared with controls (MD = −1.09, 95% CI [−1.82 to −0.36], adjusted P < .05, Supplemental Data).
The 1-way ANCOVA results compared regional MPF values in the trigeminal nerve root between individuals with PTN (affected side) and healthy controls. A, MPF values at the REZ (a medial segment of TN cisternal portion). B, values in the central segment. C, values in the lateral segment. D, average MPF value across the cisternal part of the trigeminal nerve. *P < .05; **P < .01; ***P < .001.
Comparison of the Regional MPF Values in the Affected versus Contralateral Trigeminal Nerve Root in Patients with PTN
The average MPF value was significantly lower in the affected nerve root of patients with PTN than in the contralateral nerve root (MD = −0.77, 95% CI [−1.37 to −0.18], P < .05, FDR-corrected, Fig 5B). Specifically, we detected a reduction in MPF in the REZ region (MD = −1.44, 95% CI [−2.19 to −0.69], adjusted P < .001, Fig 5A). No statistically significant differences were found in the MPF values of the central and lateral segments between the groups (P > .1, detailed in Supplemental Data).
The 1-way ANCOVA results compared regional MPF values in the affected and contralateral trigeminal nerve root in individuals with PTN. A, MPF values at the REZ (a medial segment of TN cisternal portion), while (B) represents the average MPF value across the cisternal part of the trigeminal nerve. * P < .05; *** P < .001.
Comparisons of Regional MPF Values in Patients with the Classic versus Idiopathic Subtype of PTN
No statistically significant differences were found in the MPF values of the medial, central, and lateral segments between the groups (P > .1 for all comparisons, Supplemental Data).
Correlations between MPF Values in the Affected Nerve Root and Sindou Grade
A negative correlation was shown between MPF values within the REZ segment of the affected trigeminal nerve root and the Sindou grade (R = −0.35, 95% CI [−0.59 to −0.05], adjusted P < .05; Fig 6A). Additionally, there was a negative association between MPF values within the central segment of the cisternal part of the affected trigeminal nerve root and Sindou grade (R = −0.29, 95% CI [−0.54 to −0.03], adjusted P < .05; Fig 6B). There was also a trend toward a negative association between the average MPF values of the cisternal part of the affected trigeminal nerve root and Sindou grade (R = −0.27, 95% CI [−0.54 to 0.02], adjusted P = .051; Fig 6C). No correlation was found between MPF values in the lateral segment and the Sindou grade (P > .1, Supplemental Data).
Relationship (Spearman correlation) between MPF values for the cisternal part of the trigeminal nerve and Sindou grade: (A) at the REZ (a medial segment of TN cisternal portion); (B) at the central segment; (C) average MPF values. The gray zone around the black line represents the 95% CI for the correlation coefficient.
Correlations among the MPF Values in the Affected Nerve Root, Nerve Root Atrophy, Disease Duration, and Facial Pain Intensity
No correlations were revealed between MPF values and the trigeminal nerve root thickness (P > .1 for all comparisons, Supplemental Data). Additional ANCOVA analysis did not reveal statistically significant differences in MPF values within the trigeminal nerve between REZ and lateral neurovascular compression (P > .1 for all comparisons, Supplemental Data). No notable correlations were found between regional MPF values and BPI scores (P > .1 for all comparisons, Supplemental Data). Similarly, there were no significant correlations between regional MPF values and disease duration (P > .1 for all comparisons, Supplemental Data).
DISCUSSION
In the current study, we focused on comparing MPF values in the cisternal part of the trigeminal nerve root between patients with PTN and controls. A statistically significant decrease in MPF values was found within the cisternal part of the trigeminal nerve in patients with PTN compared with healthy controls. The most noticeable microstructural changes were observed in the proximal region of the trigeminal nerve root, particularly in the REZ segment, with less pronounced changes observed in the central segment of the cisternal part. Given that MPF mapping has high sensitivity and specificity to myelin,25 these results confirm the assumption that myelin damage is an important part of PTN pathobiology.
To the best of our knowledge, this is the first effort to apply fast MPF mapping to trigeminal nerve root imaging, as well as PTN research in general. Some previous articles have explored the effectiveness of various myelin-sensitive imaging techniques for cranial nerves,40,41 but with a primary emphasis on the optic nerve. While some research groups have attempted to assess the brain myelin content in individuals with primary and secondary TN by using the T1-weighted/T2-weighted signal intensity ratio, they did not specifically examine the trigeminal nerve.42,43
Overall, our findings align with those reported in the prior studies that utilized DTI in PTN. A common finding in patients with PTN is a reduction in fractional anisotropy in the affected nerve root, which is considered an indirect marker of demyelination.14,16,17,44 Additionally, the studies have demonstrated that these alterations may improve after neurovascular decompression surgery.16 However, it is important to note that DTI has limited myelin specificity,19 and the decrease in FA values could also be attributed to other conditions within the affected nerve root, such as edema. Another drawback of DTI is its relatively low spatial resolution, with voxel sizes typically exceeding 1.5–2 mm3 for 3T systems; this limitation could hinder the visualization of small structures such as the trigeminal nerve root, particularly in cases where thinning or atrophy occurs due to chronic disease. Nevertheless, previous studies have not specifically concentrated on the trigeminal nerve root when comparing DTI and MPF techniques.19 Further exploration in this area is warranted, given the consistent findings in our study and previous research implementing DTI in patients with PTN.
In the present study, we found a negative correlation between MPF values within the REZ segment of the affected trigeminal nerve root and the Sindou grade. The root entry zone is known to be highly susceptible to microstructural damage in cases of neurovascular compression due to its unique microanatomy,5 and myelin impairment in this specific segment is a well-documented aspect of the pathobiology of this disease.6,8 Our study also revealed negative associations between MPF values in the central segment, as well as a trend toward a negative association between average MPF values in the cisternal part and Sindou grade, indicating that increased compression severity could lead to demyelination that extends beyond the REZ.
Our study did not identify statistically significant correlations between MPF values within the affected trigeminal nerve, the intensity of pain, or the duration of the disease. Currently, there are conflicting data in the literature regarding the relationship between trigeminal nerve structural integrity and pain intensity. Some authors reported associations17 and others did not.44 This may be attributed to the challenges in accurately measuring disease severity and the presence of various other factors that could affect nerve root structural integrity in individuals with PTN.
Interestingly, our findings showed a reduction in MPF values in the contralateral trigeminal nerve root in patients with PTN compared with healthy controls at the central portion of the TN cisternal segment. One possible explanation is the widespread distribution of sensitization processes throughout the entire trigeminal system, a concept that has been well documented4. For example, Obermann et al45 reported a notable increase in pain-related evoked potentials in all divisions of the trigeminal nerve on both symptomatic and nonsymptomatic sides.
Together, the results of this study provide direct in vivo evidence supporting the occurrence of myelin abnormality in the trigeminal nerve root in patients with PTN. Our results suggest that MPF may serve as a useful quantitative biomarker for assessing myelin content within the cisternal part of the trigeminal nerve in a clinical setting. Exploring the impact of microstructural nerve damage on the development and progression of PTN, as well as its clinical significance, could be a potential avenue for further investigation. It is also interesting to assess other components of the pain processing system, as well as alterations in myelination in the entire brain in patients with PTN by using the fast MPF mapping method.
This study has several limitations. First, we chose not to categorize the PTN group into classic and idiopathic subgroups and did not introduce classification based on the type of vessel that causes compression due to limitations in our sample size that precluded further division. This limitation prevents us from drawing any definitive conclusions beyond the initial observations. In addition, all patients with PTN enrolled in the study underwent pharmacotherapy, often for extended periods of time, which may have influenced the demyelination and remyelination processes. There is evidence suggesting that long-term use of carbamazepine and gabapentin could lead to neurodegenerative changes in the brain,46 but it remains unclear whether this could impact myelin. Future research should focus on including drug-naive patients to further explore this issue. Furthermore, our study did not include follow-up data. However, exploring the possible recovery of myelin content after surgical intervention is crucial. Therefore, this area warrants further investigation. Finally, this study is based on the manual ROI analysis, which is prone to interobserver errors. Future studies may benefit from combining MPF mapping with recently developed automated trigeminal nerve segmentation algorithms.47⇓-49
CONCLUSIONS
In summary, our preliminary results suggest that MPF could serve as a new neuroimaging biomarker of trigeminal nerve root impairment in patients with PTN and could enable noninvasive detection of nerve root demyelination. A reduction in myelin content in the REZ segment was associated with the severity of neurovascular compression, indicating the clinical importance of myelin damage in this condition. Our findings emphasize the importance of myelin-related changes in understanding the underlying mechanisms of TN. From a methodologic perspective, this research lays the groundwork for potential applications of the fast MPF mapping method in future clinical investigations of TN and other conditions involving cranial nerves.
Footnotes
Software for MPF map reconstruction was distributed under support of the NIH High-Impact Neuroscience Research Resource grant R24NS104098.
Disclosure forms provided by the authors are available with the full text and PDF of this article at www.ajnr.org.
References
- 1.
- 2.
- 3.
- 4.
- 5.
- 6.
- 7.
- 8.
- 9.
- 10.
- 11.
- 12.
- 13.
- 14.
- 15.
- 16.
- 17.
- 18.
- 19.
- 20.
- 21.
- 22.
- 23.
- 24.
- 25.
- 26.
- 27.
- 28.
- 29.
- 30.
- 31.
- 32.
- 33.
- 34.
- 35.
- 36.
- 37.
- 38.
- 39.
- 40.
- 41.
- 42.
- 43.
- 44.
- 45.
- 46.
- 47.
- 48.
- 49.
- Received June 8, 2024.
- Accepted after revision August 21, 2024.
- © 2025 by American Journal of Neuroradiology