Graphical Abstract
Abstract
BACKGROUND AND PURPOSE: Accelerated and blood-suppressed postcontrast 3D intracranial vessel wall MRI (IVW) enables high-resolution rapid scanning but is associated with low SNR. We hypothesized that a deep-learning (DL) denoising algorithm applied to accelerated, blood-suppressed postcontrast IVW can yield high-quality images with reduced artifacts and higher SNR in shorter scan times.
MATERIALS AND METHODS: Sixty-four consecutive patients underwent IVW, including conventional postcontrast 3D T1-sampling perfection with application-optimized contrasts by using different flip angle evolution (SPACE) and delay alternating with nutation for tailored excitation (DANTE) blood-suppressed and CAIPIRINHIA-accelerated (CAIPI) 3D T1-weighted TSE postcontrast sequences (DANTE-CAIPI-SPACE). DANTE-CAIPI-SPACE acquisitions were then denoised by using an unrolled deep convolutional network (DANTE-CAIPI-SPACE+DL). SPACE, DANTE-CAIPI-SPACE, and DANTE-CAIPI-SPACE+DL images were compared for overall image quality, SNR, severity of artifacts, arterial and venous suppression, and lesion assessment by using 4-point or 5-point Likert scales. Quantitative evaluation of SNR and contrast-to-noise ratio (CNR) was performed.
RESULTS: DANTE-CAIPI-SPACE+DL showed significantly reduced arterial (1 [1–1.75] versus 3 [3–4], P < .001) and venous flow artifacts (1 [1–2] versus 3 [3–4], P < .001) compared with SPACE. There was no significant difference between DANTE-CAIPI-SPACE+DL and SPACE in terms of image quality, SNR, artifact ratings, and lesion assessment. For SNR ratings, DANTE-CAIPI-SPACE+DL was significantly better compared with DANTE-CAIPI-SPACE (2 [1–2], versus 3 [2–3], P < .001). No statistically significant differences were found between DANTE-CAIPI-SPACE and DANTE-CAIPI-SPACE+DL for image quality, artifact, arterial blood and venous blood flow artifacts, and lesion assessment. Quantitative vessel wall SNR and CNR median values were significantly higher for DANTE-CAIPI-SPACE+DL (SNR: 9.71, CNR: 4.24) compared with DANTE-CAIPI-SPACE (SNR: 5.50, CNR: 2.64) (P < .001 for each), but there was no significant difference between SPACE (SNR: 10.82, CNR: 5.21) and DANTE-CAIPI-SPACE+DL.
CONCLUSIONS: DL denoised postcontrast T1-weighted DANTE-CAIPI-SPACE accelerated and blood-suppressed IVW showed improved flow suppression with a shorter scan time and equivalent qualitative and quantitative SNR measures relative to conventional postcontrast IVW. It also improved SNR metrics relative to postcontrast DANTE-CAIPI-SPACE IVW. Implementing DL denoised DANTE-CAIPI-SPACE IVW has the potential to shorten protocol time while maintaining or improving the image quality of IVW.
ABBREVIATIONS:
- CAIPI
- controlled aliasing in parallel imaging
- CNR
- contrast-to-noise ratio
- DANTE
- delay alternating with nutation for tailored excitation
- DL
- deep learning
- ICC
- intraclass correlation coefficient
- IVW
- intracranial vessel wall MRI
- SOC
- standard of care
- SPACE
- sampling perfection with application-optimized contrasts by using different flip angle evolution
SUMMARY
PREVIOUS LITERATURE:
High-quality IVW requires a balance of adequate SNR, high spatial resolution, and effective blood suppression, with long scan times and high signal demand serving as major challenges to clinical workflows. Accelerated and blood-suppressed postcontrast 3D intracranial vessel wall MRI (DANTE-CAIPI-SPACE) has been used for more efficient IVW scanning, but the technique suffers from low SNR. Recent studies demonstrated that DL denoising techniques improved image quality and SNR in various imaging contexts, including brain MRI and proton-density IVW.
KEY FINDINGS:
DL-denoised postcontrast DANTE-CAIPI-SPACE IVW significantly improved arterial and venous blood flow suppression and artifact reduction compared with conventional SPACE. In addition, it achieved significantly higher SNR ratings and quantitative SNR values relative to DANTE-CAIPI-SPACE and was comparable to SPACE.
KNOWLEDGE ADVANCEMENT:
Implementation of DL DANTE-CAIPI-SPACE IVW has the potential to shorten protocol time while maintaining or improving the image quality of vessel wall imaging protocols.
Early and accurate diagnosis and characterization of intracranial vasculopathies, including intracranial atherosclerotic disease and inflammatory vasculopathies, can help direct patient management for improved patient outcomes.1,2 Conventional luminal imaging modalities are the reference standard for diagnosis and characterization; however, they have limited accuracy for intracranial vasculopathy differentiation.3,4
Intracranial vessel wall MRI (IVW) is an increasingly utilized technique, with over 55% of the American Society of Neuroradiology member groups utilizing it on a regular basis.5 IVW facilitates the direct visualization and evaluation of arterial walls, improving pathologic lesion assessment.6,7 Compared with traditional imaging approaches, IVW can enhance diagnostic accuracy for intracranial vasculopathies.8⇓⇓⇓-12 IVW has also shown value in vasculopathy characterization, defining vulnerable vasculopathy features that can portend increased risk to patients.3,9,13⇓⇓⇓⇓-18
The main challenges of IVW include long scan times and increased signal demands, primarily due to the need for high spatial resolution. Extended scan times may result in patient discomfort, motion degradation, and limitations in routine clinical scanning feasibility.9 In addition, imaging artifacts, including blood flow and CSF artifacts from nonsuppression, can contribute to interpretation challenges and misdiagnoses.19,20
To address these issues, various acceleration techniques have been developed and utilized in IVW. One such technique is controlled aliasing in parallel imaging (CAIPI), optimized for 3D acquisitions and enabling higher acceleration rates.19 Delay alternating with nutation for tailored excitation (DANTE) is a blood-suppression technique that has been used for reducing CSF and blood flow artifacts in IVW.21 Sannananja et al19 demonstrated that 3D T1-DANTE-CAIPI-SPACE (sampling perfection with application-optimized contrasts by using different flip angle evolution) IVW had better image quality for lesion detection than postcontrast 3D T1-SPACE but suffered from lower SNR.
In this study, we applied a deep learning (DL)-based image denoising algorithm to DANTE-CAIPI blood-suppressed and accelerated T1 postcontrast (DANTE-CAIPI-SPACE) acquisitions. We compared qualitative and quantitative image quality with the source DANTE-CAIPI-SPACE acquisitions and SPACE. We hypothesized that the combination of DL-based denoising combined with DANTE-CAIPI (DANTE-CAIPI-SPACE+DL) could maintain the advantages of DANTE-CAIPI-SPACE over SPACE while also improving SNR, thus providing high image quality with reduced artifacts in a shortened scan time.
MATERIALS AND METHODS
Patient Selection
This study received approval from the University of Washington institutional review board, and all participants provided informed consent. Imaging and clinical data from 64 consecutive patients who had IVW examinations between May 2017 and April 2019 were extracted. Inclusion criteria involved adult patients older than 18 years who underwent IVW with and without contrast for the evaluation of suspected intracranial vascular diseases, including evaluation of cerebral aneurysm, intracranial atherosclerosis, Moyamoya disease, and vasculitis or for posttreatment aneurysm assessment. Exclusion criteria included cases with incomplete imaging data sets (lack of postcontrast SPACE, DANTE-CAIPI-SPACE, or DANTE-CAIPI-SPACE+DL).
Imaging Protocols
Scanning was performed on a 3T Prisma MR scanner (Siemens Healthineers) by using a 64-channel neurovascular coil. Sequences acquired included TOF-MRA, T1-SPACE precontrast, T2-SPACE, and postcontrast conventional T1-SPACE and T1-DANTE-CAIPI-SPACE (DANTE-CAIPI-SPACE). After IV contrast injection of 0.1 mmol/kg Multihance (Bracco), T2-SPACE was scanned, followed sequentially by T1-SPACE and T1-DANTE-CAIPI-SPACE performed in a randomized order. The alternation of the postcontrast acquisition order was performed to mitigate order bias or any potential effects on image quality related to the length of the postcontrast delay. The parameters for the sequences relevant to this study are listed in Table 1.
Imaging acquisition parameters
A default commercially available AI-driven image enhancement software, SubtleMR Version 2.4 (Subtle Medical, with FDA 510(K) clearance under K223623), was utilized on DANTE-CAIPI-SPACE sequences to enhance image quality. This software leverages an unrolled deep convolutional neural network trained on a vast data set of low- and high-quality image pairs from multiple manufacturers, institutions, and magnetic field strengths. Following the application of this AI-based algorithm, a new set of images with the same nominal resolution as the DANTE-CAIPI-SPACE sequence was produced.
Qualitative Image Analysis
A senior board-certified neuroradiologist (M.M.-B.) and a second rater (G.C.) with 19 and 13 years of neurovascular imaging review experience, respectively, independently evaluated the cases. Both raters were blinded to patient and sequence information. For each case, the sequence descriptions and labels were removed, and the SPACE, DANTE-CAIPI-SPACE, and DANTE-CAIPI-SPACE+DL were reviewed in parallel by using RadiAnt DICOM viewer (Medixant) with ratings performed for each acquisition. The rating scales used in this study were those used in a prior study.19 The reviewers rated overall image quality, qualitative SNR, arterial blood suppression, venous blood suppression, and lesion assessment by using a 4-point Likert scale. The presence and impact of artifacts were rated on a 5-point scale modified from a previously developed scale.22 The image review score details are listed in Table 2.
Image reviewing scores
Quantitative Image Analysis
Quantitative evaluation was performed by a separate radiologist (M.K., with 6 years of neurovascular imaging experience). For each case, a region of interest was manually outlined on selected images from each type of postcontrast sequence: SPACE, DANTE-CAIPI-SPACE, and DANTE-CAIPI-SPACE+DL. ROIs were applied to both normal and diseased vessel segments. For patients being evaluated for intracranial atherosclerosis or vasculitis, the ROIs were positioned on the arterial wall lesions. For scans performed after aneurysm coiling, ROIs were placed on random arterial segments selected from either the posterior or anterior circulatory systems. Two separate outlines were drawn, one tracing the junction of the vessel wall and the lumen (inner contour) and the other at the boundary of the vessel wall and adjacent tissues (outer wall contour), by using a freehand tool in Horos software (Lesser General Public License, Version 3 [LGPL-3.0]) (Fig 1). Average signal intensity and standard deviation of lumen and wall were calculated. For comparative analysis, ROIs (3 mm in diameter) were placed on normal-appearing cortical gray matter located in proximity to the vessel being quantitatively assessed. The wall SNR was calculated as mean signal/standard deviation, where mean signal is the average signal of the vessel wall, and standard deviation of noise was measured as SD within the ROI placed in the adjacent cortex. The contrast-to-noise ratio (CNR) between wall and lumen was calculated as CNR = (wall mean signal–lumen mean signal)/cortex SD = (wall SNR–lumen SNR).23 To assess interrater reliability for the quantitative comparison, a second rater (M.H.M., with 1 year of radiology experience) analyzed 30 randomly selected cases by using the same methodology and location. Additionally, the first rater repeated the measurements on this subset to evaluate intrarater reproducibility.
ROIs were manually drawn on selected images from each type of post contrast sequence. Two separate outlines were drawn: one tracing the junction of the vessel wall and the lumen (inner contour) and the other at the boundary of the vessel wall and adjacent tissues (outer wall contour).
Statistical Analysis
All statistical analyses were performed by using statsmodels 0.13.5 with Python 3.10. Both qualitative and quantitative assessments were summarized as median and interquartile ranges. The normality of the data was assessed using the Shapiro-Wilk test and further confirmed by visual inspection of histograms and Q-Q plots, and not all data were normally distributed.
We employed bootstrapping methods to test the median of the differences between paired observations. Effect sizes were quantified by using Cliff's δ. CIs were reported for the quantitative measurements, such as wall SNR and CNR. Additionally, to control the risk of type I errors due to multiple comparisons, we applied the Bonferroni correction, setting the threshold for statistical significance at P < .05/3 = .017.
To evaluate agreement of the qualitative assessment, the intraclass correlation coefficient (ICC) was obtained from a 2-way mixed model, with 95% CI calculated by bootstrapping. An ICC value of less than 0.4 was considered poor agreement, a value of 0.4–0.75 was considered good agreement, and a value of 0.75 or greater was considered excellent agreement.
RESULTS
A total of 64 subjects were included in the study, ranging in age from 22 to 76 years, with a median age of 53 years. The most common clinical diagnosis was posttreatment aneurysm (25 patients) and intracranial atherosclerosis (20 patients). The demographic and clinical features of the subjects are listed in the Online Supplemental Data.
Qualitative Imaging Assessment
Qualitative comparisons between SPACE and DANTE-CAIPI-SPACE+DL revealed significant differences in several areas, expressed in median values and interquartile range (1st quartile–3rd quartile) (Table 3). DANTE-CAIPI-SPACE+DL showed significantly reduced arterial (1 [1–1.75] versus 3 [3–4], P < .001) (Figs 2 and 3) and venous flow artifacts (1 [1–2] versus 3 [3–4], P < .001) (Fig 4) compared with SPACE. However, there were no significant differences between DANTE-CAIPI-SPACE+DL and SPACE in terms of image quality, SNR, artifact ratings, and lesion assessment.
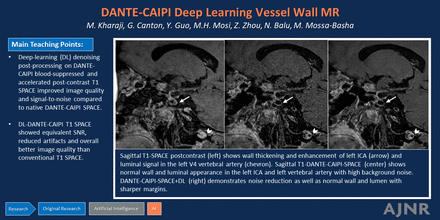
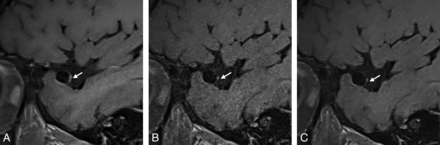
Sagittal T1-SPACE postcontrast (A) shows wall thickening and enhancement of left ICA (arrow) and luminal signal in the left V4 vertebral artery (chevron). Sagittal T1-DANTE-CAIPI-SPACE (B) shows normal wall and luminal appearance in the left ICA and left vertebral artery with high background noise. DANTE-CAIPI-SPACE+DL (C) demonstrates noise reduction as well as normal wall and lumen with sharper margins.
Sagittal T1-SPACE postcontrast (A) shows enhancement of right MCA aneurysm (arrow) along its posterior and inferior walls, with partial enhancement along the anterior and superior walls. Sagittal T1-DANTE-CAIPI-SPACE postcontrast (B) of the same aneurysm shows reduced wall enhancement relative to T1-SPACE with residual punctate focus of enhancement along the posterior wall (arrow). DANTE-CAIPI-SPACE+DL (C) demonstrates noise reduction as well as sharper margins compared with DANTE-CAIPI-SPACE, with similar pseudo enhancement suppression as seen on DANTE-CAIPI-SPACE.
Sagittal T1-SPACE postcontrast (A) shows eccentric wall thickening and enhancement of left M1 MCA mimicking a plaque (arrow). Sagittal T1-DANTE-CAIPI-SPACE (B) shows normal wall of the left M1 MCA and an adjacent vein that represents artifact on sagittal T1-SPACE (A). DANTE-CAIPI-SPACE+DL (C) demonstrates noise reduction as well as venous blood suppression.
Performance comparison of SPACE versus DANTE-CAIPI-SPACE+DL, values expressed as median (1st quartile, 3rd quartile). P values were reported to test the median of the differences between paired observations by bootstrap. Effect sizes were quantified by using Cliff's δ
Regarding SNR, DANTE-CAIPI-SPACE+DL was significantly superior to DANTE-CAIPI-SPACE (2 [1–2] versus 3 [2–3], P < .001). There were no statistically significant differences between DANTE-CAIPI-SPACE and DANTE-CAIPI-SPACE+DL for image quality, artifact, arterial blood and venous blood flow artifacts (Fig 5), and lesion assessment. Table 4 provides a complete comparison between DANTE-CAIPI-SPACE and DANTE-CAIPI-SPACE+DL.
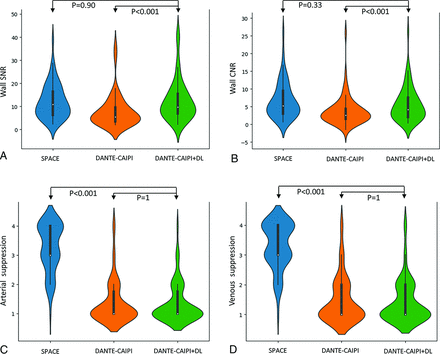
Median values and distribution of image quality metrics across SPACE, DANTE-CAIPI-SPACE, and DANTE-CAIPI-SPACE+DL sequences. A and B, Significant difference in quantitative SNR and CNR values between DANTE-CAIPI-SPACE+DL and DANTE-CAIPI-SPACE and no significant difference between DANTE-CAIPI-SPACE+DL and SPACE. C and D, Significantly better ratings for arterial and venous blood suppression in DANTE-CAIPI-SPACE+DL compared with SPACE, and no significant difference between DANTE-CAIPI-SPACE+DL and DANTE-CAIPI-SPACE.
Performance comparison of DANTE-CAIPI-SPACE versus DANTE-CAIPI-SPACE+DL, values expressed as median (1st quartile, 3rd quartile). P values were reported to test the median of the differences between paired observations by bootstrap. Effect sizes were quantified by using Cliff's δ
Quantitative Assessment
Wall SNR.
Vessel wall SNR median values for SPACE, DANTE-CAIPI-SPACE, and DANTE-CAIPI-SPACE+DL were 10.82 (6.22–16.43), 5.50 (3.52–9.57), and 9.71 (6.99–15.53), respectively. There was a statistically significant difference between DANTE-CAIPI-SPACE and DANTE-CAIPI-SPACE+DL (P < .001, 95% CI: −5.01, −3.03), but no significant difference between SPACE and DANTE-CAIPI-SPACE+DL (P = .90, 95% CI: −1.41, 2.1).
Wall CNR.
Vessel wall CNR median values for SPACE, DANTE-CAIPI-SPACE, and DANTE-CAIPI-SPACE+DL were 5.21 (2.99–9.49), 2.64 (1.50–4.37), and 4.24 (2.10–7.65), respectively. A significant difference was observed between DANTE-CAIPI-SPACE and DANTE-CAIPI-SPACE+DL (P < .001, 95% CI: −1.91, −0.48), but there was no significant difference between SPACE and DANTE-CAIPI-SPACE+DL (P = .33, 95% CI: −0.14, 1.96) (Fig 4).
Agreement Analysis
Interrater agreement for the qualitative parameters was good, ranging from 0.618 to 0.769 (Table 5). For quantitative wall SNR, the intrarater ICC was 0.909 (0.860, 0.942), and interrater ICC was 0.752 (0.633, 0.836). For wall CNR, the intrarater ICC was 0.911 (0.863, 0.943), and interrater ICC was 0.763 (0.649, 0.843).
Interrater agreement, values expressed as median (1st quartile, 3rd quartile) for each reviewer and ICC value (95% CI)
DISCUSSION
In this study, we applied a DL-based image denoising algorithm to DANTE-CAIPI-SPACE blood-suppressed and accelerated T1-weighted postcontrast acquisitions and compared qualitative and quantitative image quality with DANTE-CAIPI-SPACE and conventional 3D SPACE IVW. DANTE-CAIPI-SPACE+DL demonstrated significant improvements in arterial and venous blood flow suppression when compared with conventional SPACE. In addition, it achieved significantly higher SNR ratings and quantitative SNR values relative to DANTE-CAIPI-SPACE and comparable with SPACE. These findings suggest that the integration of DL denoising and DANTE-CAIPI-SPACE image acceleration and blood suppression can improve image quality in a shortened scan time to potentially facilitate improved MRI workflow efficiency.
Achieving the best results from IVW demands a combination of adequate SNR, superior spatial resolution, and effective blood suppression to clearly visualize the vessel wall. Presently, the most utilized sequences for 3D IVW are T1-weighted fast spin-echo sequences with variable refocusing flip angles.7 These sequences face challenges with ensuring complete blood suppression, however, particularly following the administration of intravenous gadolinium, which shortens the blood's time to inversion. The necessity for high-resolution imaging often compromises SNR, resulting in elevated background noise and increased luminal signal.24,25 Beyond the technical necessities, extended acquisition times pose a barrier to the broad adoption of IVW in demanding clinical settings. Longer scan times also result in increased random motion, especially in critically ill and noncooperative patients, leading to image quality degradation. In a previous study,19 postcontrast DANTE-CAIPI-SPACE IVW exhibited better qualitative ratings for image artifacts, reduced motion, and enhanced blood suppression, with scan time reduction of 38% compared with conventional IVW; however, the SNR ratings were lower both qualitatively and quantitatively. In the current study, we used the same data set and integrated DL to denoise the DANTE-CAIPI-SPACE acquisitions, leading to improved SNR relative to the native DANTE-CAIPI-SPACE acquisition.
There has been a recent increased focus on DL denoising applications for neuroimaging.26,27 Yamamoto et al28 applied DL-based reconstruction to time-accelerated 3D-FLAIR in 28 patients with multiple sclerosis and found that DL denoising reconstruction significantly improved subjective and quantitative image quality metrics, including SNR and white matter lesion edge compared with accelerated 3D-FLAIR acquisitions without denoising. Rudie et al29 evaluated AI-based image denoising on 3D volumetric time-accelerated brain MRI (45% faster) in 32 patients and compared it with standard of care (SOC) 3D brain MRI. They found AI-denoised sequences with a 45% scan time reduction were noninferior in overall image quality metrics and provided qualitative SNR improvement compared with SOC scans. These findings support the idea that DL denoising can facilitate equivalent image quality with accelerated scans in other acquisitions, as our findings show when applied to 3D IVW.
Eun et al30 applied 2 DL methods (self-supervised and unsupervised) on compressed SENSE 3D proton-density IVW in 18 healthy subjects and found that DL output images had less noise and higher SNR than the conventional acquisition. Their results show the potential of IVW image quality improvements with DL algorithms, a potential that we have proved to be true also in patients with intracranial vasculopathies by improving the SNR in shortened IVW acquisitions.
The significant improvement in reduction of arterial and venous blood artifacts with DANTE-CAIPI-SPACE+DL confirms our hypothesis that DL denoising can effectively improve image quality and reduce scan time while also preserving SNR relative to full conventional IVW acquisitions and improve over the baseline DANTE-CAIPI-SPACE accelerated and blood-suppressed acquisition. However, our study had several limitations. First, our analysis encompassed a relatively small data set of cases, indicating a need for a larger data set to further validate these findings. Second, our study was confined to images obtained from a single MRI vendor at a single institution and by using a single DL denoising algorithm, which may limit generalizability of the results. Application and comparison between various algorithms by using data from multiple vendors may better indicate additional and/or optimal approaches for improved image quality.
CONCLUSIONS
DL denoised postcontrast T1-weighted DANTE-CAIPI-SPACE accelerated and blood-suppressed IVW had improved image quality qualitative scores with a shorter scan time and equivalent qualitative and quantitative SNR measures relative to conventional postcontrast IVW and improved SNR metrics relative to postcontrast DANTE-CAIPI-SPACE IVW. Implementation of DL DANTE-CAIPI-SPACE IVW has the potential to shorten protocol time while maintaining or improving the image quality of vessel wall imaging protocols.
Footnotes
This research has been supported by the National Institutes of Health under Grants R01-NS125635 and R01-NS092207.
Disclosure forms provided by the authors are available with the full text and PDF of this article at www.ajnr.org.
References
- 1.
- 2.
- 3.
- 4.
- 5.
- 6.
- 7.
- 8.
- 9.
- 10.
- 11.
- 12.
- 13.
- 14.
- 15.
- 16.
- 17.
- 18.
- 19.
- 20.
- 21.
- 22.
- 23.
- 24.
- 25.
- 26.
- 27.
- 28.
- 29.
- 30.
- Received May 2, 2024.
- Accepted after revision July 14, 2024.
- © 2025 by American Journal of Neuroradiology