Abstract
BACKGROUND AND PURPOSE: Hemangioblastoma is a rare vascular tumor that occurs within the central nervous system in children. Differentiating hemangioblastoma from other posterior fossa tumors can be challenging on imaging, and preoperative diagnosis can change the neurosurgical approach. We hypothesize that a “lightbulb sign” on the arterial spin-labeling (ASL) sequence (diffuse homogeneous intense hyperperfusion within the solid component of the tumor) will provide additional imaging finding to differentiate hemangioblastoma from other posterior fossa tumors.
MATERIALS AND METHODS: In this retrospective comparative observational study, we only included pathology-proved cases of hemangioblastoma, while the control group consisted of other randomly selected pathology-proved posterior fossa tumors from January 2022 to January 2024. Two blinded neuroradiologists analyzed all applicable MRI sequences, including ASL sequence if available. ASL was analyzed for the lightbulb sign. Disagreements between the radiologists were resolved by a third pediatric neuroradiologist. χ2 and Fisher exact test were used to analyze the data.
RESULTS: Ninety-five patients were enrolled in the study; 57 (60%) were boys. The median age at diagnosis was 8 years old (interquartile range: 3−14). Of the enrolled patients, 8 had hemangioblastoma, and 87 had other posterior fossa tumors, including medulloblastoma (n = 31), pilocytic astrocytoma (n = 23), posterior fossa ependymoma type A (n = 16), and other tumors (n = 17). The comparison of hemangioblastoma versus nonhemangioblastoma showed that peripheral edema (P = .02) and T2-flow void (P = .02) favor hemangioblastoma, whereas reduced diffusion (low ADC) (P = .002) and ventricular system extension (P = .001) favor nonhemangioblastoma tumors. Forty-two cases also had ASL perfusion sequences. While high perfusion favors hemangioblastoma (P = .03), the lightbulb sign shows a complete distinction because all the ASL series of hemangioblastoma cases (n = 4) showed the lightbulb sign, whereas none of the nonhemangioblastoma cases (n = 38) showed the sign (P < .001).
CONCLUSIONS: Lightbulb-like intense and homogeneous hyperperfusion patterns on ASL are helpful in diagnosing posterior fossa hemangioblastoma in children.
ABBREVIATIONS:
- ASL
- arterial spin-labeling
- DCE
- dynamic contrast-enhanced
- IQR
- interquartile range
- VHL
- Von Hippel Lindau
- WHO
- World Health Organization
SUMMARY
PREVIOUS LITERATURE:
Hemangioblastoma is a rare, highly vascular tumor in children. Differentiating hemangioblastoma from other posterior fossa tumors can be challenging with conventional MRI sequences. We hypothesize that a lightbulb-like diffuse hyperintense signal on the ASL sequence will provide additional imaging findings to differentiate hemangioblastoma from other posterior fossa tumors.
KEY FINDINGS:
Two blinded neuroradiologists accurately identified 4 hemangioblastomas among 42 pediatric posterior fossa tumors by using the “lightbulb sign” criteria on ASL perfusion imaging. While conventional qualitative analyses of ASL perfusion were still useful in diagnosing hemangioblastoma, the implementation of the lightbulb sign criteria helped distinguish hemangioblastoma from other posterior fossa tumors with high perfusion.
KNOWLEDGE ADVANCEMENT:
The markedly elevated homogeneous signal of the tumor on ASL CBF mapping is a promising imaging characteristic of posterior fossa hemangioblastomas in children.
Hemangioblastoma is a vascular mesenchymal tumor.1 Due to its highly vascular nature, it stands with hemangioma in the vascular tumor group in the 2021 World Health Organization (WHO) Classification of Tumors of the Central Nervous System.1 Hemangioblastoma typically arises in the posterior fossa, followed by the spine, and less commonly in supratentorial locations.2⇓-4 While Von Hippel-Lindau (VHL) disease is a systemic disease with a high prevalence of CNS hemangioblastoma, the most common form of hemangioblastoma is sporadic in the adult population.4,5 Hemangioblastoma differs in the pediatric population because it mostly occurs in the spine and is commonly associated with VHL disease.6 Although hemangioblastoma is a low-grade tumor, concomitant VHL disease, solid tumor morphology, and brainstem localization are associated with poor prognosis, and the recurrence rate is higher in children.6 The cyst with a solid mural nodule appearance is the classic imaging presentation of the tumor, but it can have different morphologic patterns, including solid tumors.7 Hemangioblastomas can be further classified based on radiologic appearance into 4 groups: 1) solid, 2) extratumoral cysts, 3) intratumoral cysts, and 4) extratumoral and intratumoral cysts.8 Beyond morphology, T2-flow voids, reflecting the tumor's extensive vascularization, can favor hemangioblastoma.7 Metastases can mimic hemangioblastomas, but higher ADC values and increased perfusion on DSC and dynamic contrast-enhanced (DCE) imaging help to differentiate hemangioblastomas from metastases in adults.7,9 Pilocytic astrocytoma is another important mimic of posterior fossa hemangioblastoma in the pediatric population.10 ADC, MRS, and DSC imaging can also be useful to differentiate hemangioblastoma from those.10,11 Arterial spin-labeling (ASL) is a popular perfusion imaging method in children due to the lack of contrast necessity. ASL perfusion imaging of hemangioblastoma was previously evaluated for the adult population and found useful in distinguishing hemangioblastoma from other brain tumors, including metastases.12⇓-14 Because differential diagnosis of lesions or masses is different in the pediatric population, it is important to assess ASL perfusion performance for hemangioblastoma in children. The preoperative planning for hemangioblastomas can include embolization to decrease intraoperative complications.15
In our clinical observation, we identified a distinct ASL perfusion pattern in hemangioblastomas. This pattern is characterized by an intensely homogeneous high signal in all the tumor’s solid components, referred to as the ASL “lightbulb sign” in this study (Fig 1). Our study aims to investigate the diagnostic performance of the ASL lightbulb sign over other MRI features for distinguishing hemangioblastoma from other posterior fossa tumors in children.
A and B demonstrate an MRI of a 14-year-old boy with medulloblastoma, non-WNT/non-SHH. A, T2WI shows a large heterogeneous mass (black arrowhead) in the 4th ventricle and pushing cerebellar hemispheres. B, ASL perfusion imaging shows high perfusion (white arrowhead). Still, this study is not compatible with the ASL lightbulb sign because morphology of the high ASL signal does not match with the tumor morphology and there are also low-perfusion signals (white curved arrow) within the posterior solid part of the tumor. C and D demonstrate a 15-year-old boy with VHL disease with hemangioblastoma. C, T2WI shows a mass with internal and external cysts in the left cerebellar hemisphere (black arrow) and peripheral edema. D, ASL perfusion study demonstrates intense high perfusion in all the solid parts of the tumor and ASL high signal morphology matches with the solid component of the tumor, compatible with the lightbulb sign.
MATERIALS AND METHODS
This retrospective comparative observational study was conducted at a large academic children's hospital in the United States and followed the Strengthening the Reporting of Observational Studies in Epidemiology statements.16 The institutional review board of the hospital approved this study and waived the need for informed consent because this study relies on medical records, eliminating the need for direct participant involvement. Based on the aim of this study, we divided patients with posterior fossa tumors into 2 groups: hemangioblastoma and nonhemangioblastoma. For the group with hemangioblastoma, we included all the pathology-proved cases of hemangioblastoma within our pediatric center that had at least 1 preoperative MRI regardless of the admission date. For the group with nonhemangioblastoma, we included all the pathology-proved cases of all other posterior fossa tumors between January 2022 and January 2024 that had at least 1 preoperative MRI. We limited this group to patients diagnosed from 2022 onwards, because the latest version of the WHO Classification of Tumors of the CNS was released in late 2021.
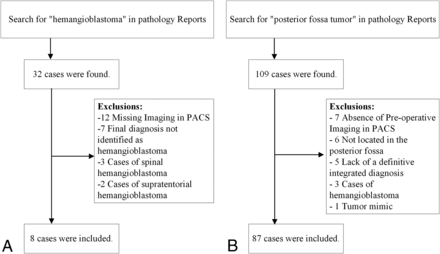
To obtain the primary list of patients, we searched pathology reports by using Illuminate InSight software (version 4.3, Softek Solutions). The search terms included “hemangioblastoma” and “posterior fossa tumor.” After obtaining a primary list, a radiologist (O.S.) reviewed pathology reports to exclude patients without a definite diagnosis of hemangioblastoma or any other posterior fossa tumors, as well as patients with spinal or cerebral hemangioblastoma. After that, the radiologist searched for the brain MRIs of these patients on the PACS, and included all cases with at least 1 preoperative brain MRI. If the patient had more than 1 preoperative MRI, the last 1 was chosen. Fig 2 shows the patient selection process.
Patient selection process.
After that, 2 neuroradiologists with 2 and 4 years of post-training experience who were blinded to the final diagnosis read all the MRIs independently and qualitatively analyzed the following variables: tumor morphology, enhancement, diffusion-restriction, necrosis, ventricular system extension, susceptibility, hydrocephalus, peripheral edema, T2-flow void, tumor count, perfusion, and presence of the lightbulb sign. The lightbulb sign is defined by the following criteria: 1) the lesion’s signal intensity on ASL CBF mapping should be remarkably higher than the cerebellar cortex; 2) the signal morphology should be diffuse and should not contain any focal hypoperfusion areas within the solid part of the tumor; and 3) the shape of the hyperperfusion should match the solid part of the tumor. After the initial assessment, any discrepancies between the 2 neuroradiologists were put into discussion to reach a consensus. If a unanimous agreement could not be reached, a pediatric neuroradiologist with 8 years of experience evaluated the case to finalize the research question.
Imaging Data Acquisition
All the examinations were done by using 1.5 or 3T MRIs (Siemens, Philips, GE Healthcare). T2-weighted series, diffusion-weighted, susceptibility-weighted, postcontrast T1 spin-echo or 3D gradient-echo, and ASL perfusion series of each MRI were selected for evaluating cases of this study. ASL perfusion techniques included 3D pulsed ASL (n = 32), 2D pulsed ASL (n = 2), and 3D pseudocontinuous ASL (n = 8). The ranges of acquisition parameters for ASL perfusion are provided in Table 1.
ASL perfusion image acquisition parameters
Statistical Analysis
IBM SPSS Statistics for Windows (version 29, IBM Corp) was used to analyze the data of this study. Nominal data were presented as frequencies and percentages, while quantitative variables were presented as medians and interquartile ranges (IQRs). Interrater agreement for dichotomous variables was assessed by using the Cohen κ for the first evaluation of 2 raters. The variables among the 2 groups were compared by using the χ2 test, Fisher exact test, and Mann-Whitney U test. A P value of less than .05 was considered the significance level.
RESULTS
After applying all the inclusion and exclusion criteria, 95 patients with a median age of 8 years (IQR: 3−14) were included in this study. Fifty-seven (60%) patients were boys, and 38 (40%) were girls. Eight (8.4%) patients had hemangioblastoma, and 87 (91.6%) had other types of posterior fossa tumors, including medulloblastoma (n = 31), pilocytic astrocytoma (n = 23), posterior fossa ependymoma type A (n = 16), and other tumors (n = 17). Comparing the demographics between these groups showed there is not a significant difference between the male-to-female ratio in these groups (P = .71). However, the age of the hemangioblastoma group was significantly higher than the nonhemangioblastoma group (15.5 [14−18.25] versus 8 [3−11], P < .001).
In the hemangioblastoma group, the most prevalent morphology was solid with external cysts (62.5%), while the most prevalent morphology in the nonhemangioblastoma group was solid with internal cysts (58.6%). The comparison of hemangioblastoma versus nonhemangioblastoma showed that peripheral edema (P = .02) and T2-flow void (P = .02) favor hemangioblastoma, whereas diffusion restriction (P = .002) and ventricular system extension (P = .001) are not consistent with hemangioblastomas. Tables 2 and 3 shows the comparison of the variables between the groups.
Comparison of demographic and morphologic features of hemangioblastoma and posterior fossa tumors other than hemangioblastoma
Comparison of demographic and morphologic features of hemangioblastoma and posterior fossa tumors other than hemangioblastoma
Of the total 95 cases, 42 also had ASL perfusion studies (4 hemangioblastoma cases and 38 nonhemangioblastoma). Of the 38 patients in the nonhemangioblastoma group, lesions of 13 (34.2%) patients, including 4 posterior fossa group A ependymoma, 3 non-WNT/non-sonic hedgehog (SHH) medulloblastoma, 2 pilocytic astrocytoma, 1 atypical teratoid/rhabdoid tumor, 1 choroid-plexus papilloma, 1 mixed germ cell tumor, and 1 neuroblastoma metastasis showed high perfusion. All 4 (100%) cases of the hemangioblastoma group also had high-perfused lesions (Fig 3). Comparing the false-positive ratios for ruling out hemangioblastoma showed that while we had a false-positive ratio of 34.2% (13/38) for high perfusion, the false-positive ratio for the lightbulb sign is 0% (0/38), showing a significant difference (P < .001). The interrater reliability for the high-perfused lesions between the first evaluation of our 2 raters was κ = 0.588.
ASL perfusion imaging of patients with pathology proved hemangioblastoma. A and B demonstrate an MRI of a 14-year-old girl with hemangioblastoma. T2WI (A) shows a mass with external cysts and a solid core (black arrow). ASL perfusion shows high intense signal in the whole solid part, in other words ASL lightbulb sign. C and D are MRI studies of a 14-year-old boy with hemangioblastoma. T2WI (C) shows a solid mass with an internal microcyst (black arrow) in the right hemisphere of the cerebellum and ASL perfusion (D) shows lightbulb sign (white arrow) at the solid core part of the mass. E and F demonstrate an MRI study of a 16-year-old girl with hemangioblastoma. T2WI (E) shows 2 masses with internal microcysts and external cysts (black arrows), ASL perfusion imaging (F) shows lightbulblike high perfusion in both of the tumor solid cores (white arrows). G and H demonstrate an MRI of a 16-year-old girl with hemangioblastoma. T2WI (G) shows an heterogeneous mass with internal and external cysts in the left cerebellar hemisphere (black arrow) and adjacent edema. ASL perfusion imaging (H) shows lightbulb sign within the tumor (white arrow).
The lightbulb sign shows a complete distinction between these groups because all the ASL series of hemangioblastoma cases showed the lightbulb sign, whereas none of the nonhemangioblastoma cases met the criteria for the lightbulb sign even if they showed high perfusion (P < .001). The interrater reliability of this sign was κ = 1.00.
DISCUSSION
Hemangioblastoma is a highly vascular benign tumor.6,7 Although more common in adults, it also occurs in children.6 The spine and posterior fossa are relatively common locations.2,4,6 The 2021 WHO Classification of Tumors of the CNS does not distinguish “pediatric hemangioblastoma” from hemangioblastoma. However, it is noteworthy that the differential diagnoses for hemangioblastoma vary between adults and children. The specific characteristics and distinctive features considered when diagnosing hemangioblastoma in adults are not entirely applicable to the pediatric population. Our goal was to identify imaging markers in differentiating hemangioblastoma from other pediatric posterior fossa tumors due to the difference in preoperative management of children with hemangioblastoma, including embolization. We clinically observed a distinct ASL perfusion pattern in hemangioblastomas. In our daily practice, we noticed a pattern of intensely and homogeneously high perfusion within all solid tumor components without any low perfusion area, which we refer to as the ASL lightbulb sign. This study investigates the validity and diagnostic value of the ASL lightbulb sign compared with other MRI findings. All the hemangioblastomas showed peripheral edema. While hemangioblastoma is a low-grade benign tumor, it is known that peritumoral edema is a common presentation in the posterior fossa.17⇓-19 Furthermore, profound surrounding edema disproportionately greater than expected for tumor size is an important feature of hemangioblastoma.8,20,21 Profound peritumoral edema and neurologic symptoms associated with hemangioblastoma typically warrant surgical resection, irrespective of solitary or multiple lesions.8,22 Our series consisted of only hemangioblastoma with surgery indication and showed 100% peritumoral edema; thus, our findings were in line with the previous literature. Another feature that we observed in all hemangioblastomas was T2-flow void. T2-flow void results from intravascular flow and intratumoral demonstration of this phenomenon is expected in highly vascular tumors, such as hemangioblastoma.7 Hemangioblastomas did not show reduced diffusion in our series and this finding was in line with previous literature.9,11 It is known that hemangioblastoma usually shows high ADC values.9,11 Diffusion characteristics can help to differentiate hemangioblastoma and metastatic masses but these values are not always useful in distinguishing it from certain posterior fossa tumors.9,11 Although peripheral edema, lack of reduced diffusion, and T2-flow void were observed in all of the hemangioblastomas in our series, almost one-half of the control group also demonstrated these features. While these findings are statistically significant and might help differentiate hemangioblastoma from other posterior fossa tumors, none is stand-alone sufficient to distinguish hemangioblastoma completely. Still, we find it valuable to define the imaging characteristics of hemangioblastoma in children as enhancing solid parts with high ADC values, T2-flow voids, and peripheral edema. In our cohort the top 3 posterior fossa tumors were medulloblastoma, pilocytic astrocytoma, and posterior fossa ependymoma type A. Because posterior fossa ependymoma type A is mostly expected in infantile age groups, a dilemma between hemangioblastoma and posterior fossa ependymoma type A is not a common clinical scenario.23 DWI can help differentiate medulloblastoma from other common posterior fossa tumors. Still there are many overlapping imaging characteristics between hemangioblastoma and other common posterior fossa tumors.24 Previous literature showed the importance of perfusion imaging in diagnosis of hemangioblastoma and based on our study ASL MRI can improve to distinguish hemangioblastoma from other posterior fossa tumors in children.10,11,25
It should be noted that DSC/DCE MR perfusion imaging has limited results in children due to difficulties in reproducing technical standards derived from adults and minimum use in children.26 Age-related normative values for relative CBV derived from MR perfusion techniques are not yet fully established for children. ASL offers many advantages that make it suitable for children, less time than DCE/DSC, as well as elimination of IV access, contrast, or radiotracer agent, in addition to decreased susceptibility artifact at skull base.27,28 These advantages of ASL over MR perfusion in children further highlight the diagnostic value of our proposed ASL lightbulb sign for accurate diagnosis of pediatric hemangioblastoma.
ASL perfusion imaging was available in 4 patients with hemangioblastoma and 38 patients with nonhemangioblastoma. Previous studies showed the high perfusion characteristics of hemangioblastoma due to its highly vascular nature.10,11,14 High signal on ASL perfusion was observed in all hemangioblastomas but we also noticed this feature in 13 other posterior fossa tumors. Our raters grouped hemangioblastomas with some other tumors when they analyzed ASL perfusion characteristics superficially as just high or not high. Interrater agreement was not high in this binary evaluation due to subjectivity associated with slight heterogeneous lesional perfusion near or just above the normal cerebellar cortex and perfusion related to nearby or coursing vessels within the tumor. We concluded that high perfusion on ASL was a typical feature of hemangioblastoma but was not specific. As we hypothesized, both blinded reviewers marked as ASL lightbulb sign all the hemangioblastomas and none of the others in the 42 ASL study pool. Our results showed detailed pattern analyses of ASL perfusion, giving a better insight into the differential diagnoses. Furthermore, intense homogeneous high signals of all the solid parts of the tumor were as precise as pathology, so-called pathognomonic, in our series. Thirteen tumors showed high perfusion but no lightbulb signs on ASL. These tumors were broad including 4 posterior fossa group A ependymoma and 3 non-WNT, non-SHH medulloblastoma. Our results suggest some tumors could mimic hemangioblastoma on ASL, but detailed analyses of the perfusion pattern to check ASL lightbulb sign can help in such cases. Moreover, ASL lightbulb sign evaluation showed higher interrater agreement than superficial qualitative ASL review.
This study has several limitations. Posterior fossa hemangioblastoma is a rare entity in children and usually this low-grade tumor is not going to resection. Because we aimed to discuss a potential pathognomonic value of a feature we had to include only pathology-proved cases. All these restrictions resulted in a small sample size. Although we believe our findings strongly indicate the pathognomonic value of the ASL lightbulb sign, we could overestimate its importance due to the small sample size. Another point is that we included only hemangioblastoma with surgery indication. Although our pathology results suggested that these tumors are classic hemangioblastoma, we still do not know whether those have specific features. Additionally, while most of our patients had 3D pulsed ASL, our cohort includes different ASL perfusion techniques. Because our analyses are qualitative, we believe the effect of technical differences is minimal, but such variations are still a factor in interpretation of perfusion. All the hemangioblastomas in our cohort had either 2D or 3D pulsed ASL. The high signal of ASL is a representation of the blood flow and is mostly independent of the technique. Still, we are not able to conclude the validity of the lightbulb sign with different ASL techniques.
We did not notice any mimic of the lightbulb sign in our cohort. On the other hand, posterior fossa lesions are not limited to tumors, and focal high perfusion is also noted in some nontumoral lesions, such as high-flow arteriovenous malformation.29 Nontumoral lesions should be excluded before implementing a lightbulb sign as a diagnostic tool. Because the tumor biology of hemangioblastoma does not change by patient age, hemangioblastomas potentially might represent lightbulb signs in different age groups. Moreover, previously published ASL figures of adult hemangioblastomas resemble our lightbulb sign.14,30 However, further studies in the adult population are necessary to confirm this postulate, and we are not able to generalize our results for the adult population. Quantitative analyses of ASL perfusion are also another common analysis of this technique but in daily practice such quantitative analyses have relatively limited usage and we wanted to provide a methodology without necessity of any measurements. Our study proved that morphologic analyses of the perfusion pattern are superior to common qualitative analyses, but we still do not know whether morphologic analyses is superior to quantitative analyses.
CONCLUSIONS
The solid part of posterior fossa hemangioblastomas in children shows enhancing nodule characteristics with T2-flow voids and nonrestricted diffusion. High signal on ASL perfusion is helpful in distinguishing hemangioblastoma from pediatric posterior fossa tumors with low perfusion. On the other hand, diffusely intense lightbulb-like signal distribution in ASL perfusion is a more specific finding of posterior fossa hemangioblastoma in children and can also be useful in distinguishing hemangioblastoma from other posterior fossa tumors with high perfusion.
Footnotes
Disclosure forms provided by the authors are available with the full text and PDF of this article at www.ajnr.org.
References
- 1.
- 2.
- 3.
- 4.
- 5.
- 6.
- 7.
- 8.
- 9.
- 10.
- 11.
- 12.
- 13.
- 14.
- 15.
- 16.
- 17.
- 18.
- 19.
- 20.
- 21.
- 22.
- 23.
- 24.
- 25.
- 26.
- 27.
- 28.
- 29.
- 30.
- Received May 2, 2024.
- Accepted after revision June 14, 2024.
- © 2024 by American Journal of Neuroradiology