Abstract
BACKGROUND AND PURPOSE: Preoperative assessment of meningioma consistency is beneficial for optimizing surgical strategy and prognosis of patients. We aim to develop a noninvasive prediction model for meningioma consistency utilizing MR elastography and DTI.
MATERIALS AND METHODS: Ninety-four patients (52 ± 22 years old, 69 women, 25 men) diagnosed with meningioma were recruited in the study. Each patient underwent preoperative T1WI, T2WI, DTI, and MR elastography. Combined MR elastography–DTI model was developed based on multiple logistic regression. Intraoperative tumor descriptions served as clinical criteria for evaluating meningioma consistency. The diagnostic efficacy in determining meningioma consistency was evaluated by using a receiver operating characteristic curve. Further validation was conducted in 27 stereotactic biopsies by using indentation tests and underlying mechanism was investigated by histologic analysis.
RESULTS: Among all the imaging modalities, MR elastography demonstrated the highest efficacy with the shear modulus magnitude (|G*|) achieving an area under the curve (AUC) of 0.81 (95% CI: 0.699–0.929). When combined with DTI, the diagnostic accuracy further increased (AUC: 0.88, 95% CI: 0.784–0.971), surpassing any technique alone. Indentation measurement based on stereotactic biopsies further demonstrated that the MR elastography–DTI model was suitable for predicting intratumor consistency. Histologic analysis suggested that meningioma consistency may be correlated with tumor cell density and fibrous content.
CONCLUSIONS: The MR elastography–DTI combined model is effective in noninvasive prediction of meningioma consistency.
ABBREVIATIONS:
- AUC
- area under the curve
- FA
- fractional anisotropy
- MD
- mean diffusivity
- ROC
- receiver operating characteristic
- SI
- signal intensity
- |G*|
- shear modulus magnitude
Meningioma is the most common type of primary intracranial tumor, originating in the arachnoid and typically exhibiting slow growth, often remaining undiagnosed for years.1,2 Surgical resection is the primary treatment for most cases of meningioma.3,4 Various intraoperative and postoperative risks are known to be associated with tumor characteristics such as consistency, adhesions, and homogeneity,5⇓⇓-8 particularly in cases in which the tumor's location presents challenges for access and resection.9 Therefore, accurate preoperative evaluation of intraoperative tumor conditions is valuable for guiding surgical strategy and risk assessment.
MRI has been widely utilized in the diagnosis of meningiomas.10,11 Research efforts have focused on leveraging both conventional and advanced MRI sequences as predictors of meningioma consistency.4,9,10,12⇓⇓-15 DTI-derived fractional anisotropy (FA) and mean diffusivity (MD) maps have been validated as predictors of meningioma consistency. Notably, FA has been shown to outperform T2WI as a predictor.16 However, certain studies have not replicated these findings.17 Additionally, both T2WI and DTI alone can reliably predict consistency only in a small number of extremely soft or firm meningiomas, significantly limiting their clinical applicability.18
MR elastography is a phase-contrast-based MRI technique that visualizes the propagation of mechanical waves in tissues, enabling noninvasive determination of tissue consistency.5,19⇓-21 While preliminary MR elastography research has been conducted in human organs such as breast,22,23 prostate,24 liver,25 and skeletal muscle,26 studies in brain MR elastography gained momentum after reports correlating tumor elasticity assessed by MR elastography with tumor consistency based on surgical findings.27,28 It has been demonstrated that the biomechanical properties of intracranial tumors measured by MR elastography significantly correlate with tumor consistency.5,17,18,29⇓-31 Nevertheless, limited studies have compared the in vivo measurement with intraoperative assessment and validated with ex vivo biomechanical testing. In addition, enhancing the predictive ability of MR elastography with other modalities remains an area of active investigation.32
In this study, we devised a multimodal approach that integrates |G*| in MR elastography and FA values in DTI to predict the consistency of intracranial meningiomas. Stereotactic biopsies as well as voxelwise analysis were performed to verify the application of the MR elastography-DTI combined model in determining intratumoral heterogeneity. Quantitative measurements of tumor consistency were obtained through indentation tests to validate the performance.
MATERIALS AND METHODS
Population
Study participants were prospectively recruited from patients diagnosed with meningioma preoperatively by experienced radiologists and confirmed by biopsies from June 2022 to October 2023. The study protocol was approved by the institutional review board, and informed consent in written form was obtained from all participants. Pathologic diagnoses were determined according to the 2021 World Health Organization Classification of Central Nervous System Tumors.33 Exclusion criteria are listed in the Online Supplemental Data.
Imaging Acquisition
MRI scanning was performed within a week before surgery. All MRI sequences including T1WI, T2WI, DTI, and MR elastography were acquired by using a 3T MRI system (uMR790, Shanghai United Imaging Healthcare). The imaging protocols are shown in the Online Supplemental Data.
Image and Data Processing
Once all MRI acquisition and construction have been completed, the MR elastography, DTI, and T2WI results were aligned to the T1WI, which was the uniform standard space. Detailed information regarding MRI construction is provided in the Online Supplemental Data.
For the delineation of brain tumors, 2 physicians independently delineated ROIs for the lesions layer by layer according to the uniform standard space images and created 3D segmentations of the tumor. The ROIs referring to tumor area were then applied on the map of each MR sequence. Any consequential discrepancies were resolved through discussion to achieve a consensus. The ROIs referring to tumor area were then applied on the map of each MR sequence. Signal intensity (SI) in T1WI, T2WI,10,17 and FA value in DTI and |G*| value in MR elastography were analyzed by ITK-SNAP software (4.0, Penn Image Computing and Science Laboratory).34 Tumor consistency was then calculated as the pixel contained in each ROI and averaged across all ROIs. The SI ratio (tumor to cerebral cortex SI) on T1WI and T2WI was used for further evaluation.35 For the SI of the cortex, an ROI (6−9 mm3) was placed within the contralateral superior frontal gyrus.36
Surgery and Intraoperative Evaluation
Two surgeon specialists in brain surgery (22 and 16 years of experience, respectively) primarily conducted all surgical resections, and their intraoperative impressions of tumor consistency served as reference standards for later analysis. Both experts were blinded to MRI data. All detailed descriptions were recorded in surgical documents. Tumor consistency was graded on the following scale: 1) soft: removed totally with suction; 2) mostly soft: removed mostly with suction; 3) medium: removed with combination of suction and ultrasonic aspirator intensity <40; 4) tenacious: removed with ultrasonic aspirator intensity between 40 and 70; and 5) hard: removed with ultrasonic aspirator intensity >70 or other sharp dissection surgical adjuncts.4,14,29 The multiple categorizations of tumor consistency were reduced to 2 categories for further model accuracy assessment, as consistent with previous studies in which tumor consistency was first graded in multiple categories and then dichotomized for model accuracy assessment.16,17,37 It is hypothesized that the efficiency of models in diagnosing tumor consistency based on dichotomies is a prerequisite for their further prediction of multiple consistency grading. In this case, “soft,” “mostly soft,” and “medium” meningiomas that can be removed by suction were categorized as “soft” (corresponding to grades 1 and 2 of Zada’s consistency grading system), whereas “tenacious” and “hard” meningiomas that can only be removed by ultrasonic aspiration were categorized as “firm” (corresponding to grades 3, 4, and 5 of Zada's consistency grading system).
For the biopsies to verify technique, specimens of the tumor were resected separately during the operation with their exact locations recorded according to the intranavigation. The consistency of each specimen was then graded individually. The acquired images from scanned MRI sequences were aligned with the intranavigation images, where each biopsy location was marked. Subsequently, the ROI was delineated for voxelwise analysis.
Indentation Measurement
A custom-built indentation device in a previous study was used to measure the tissue consistency of tumor samples ex vivo.38 Surgical specimens were taken from different regions of each tumor and the indentation measurement was conducted within 30 minutes after the sample was acquired. A ramp-hold indentation protocol was accepted in this test.39 Details of the procedure and algorithm can be found in the Online Supplemental Data. Eventually, instantaneous shear modulus ( ) was used as the result of the indentation measurement.
) was used as the result of the indentation measurement.
Pathologic Assessments
After the surgical specimens underwent indentation tests, tissue samples were collected for pathologic analysis. After formalin fixation and paraffin embedding, the tissues were stained with H&E stain and Masson stain. Tissue slides from various tumor areas were scanned digitally by using a VS200 whole-slide image scanner (Olympus) and OlyVIA software 4.1.1 at 20× magnification. Quantitative histologic parameter of tumor cellularity and fibrous content was obtained by computer-assisted analysis by using ImageJ (v6.0.0.260; Media Cybernetics).17 The average number of cell nuclei in per unit area was calculated as the cell density. Cell counting and fibrous content calculation was performed on each sample 3 times.
Statistical Methods
Statistical analyses and graphical visualization were performed by using GraphPad Prism 9.0 (GraphPad Software). The MRI variable distribution was initially assessed by using the Shapiro-Wilk test. For normally distributed data, a 2-tailed independent samples t test was conducted, while for non-normally distributed data, a Mann-Whitney U test was performed. The receiver operating characteristic (ROC) curve analysis was plotted by connecting points with a coordinate of the false-positive rate (1 − specificity) and the true-positive rate (sensitivity) for the classifiers by using various thresholds. ROC curves were compared by using the DeLong test. Areas under receiver operating characteristic curves were then analyzed to compare levels of diagnostic performance by each technique in detecting tumor consistency. Confusion matrices are also used as a statistical tool to assess the effectiveness of each predictive model. The linear relationships between TIWI, T2WI, FA, and |G*| were further assessed by using the Pearson correlation coefficient. Multiple logistic regression with 2-way interactions (consistency ∼ Intercept + FA + |G*| + FA: |G*|, β0: −21.67; β1: 43.57; β2: 0.01007; β3: −0.01946) was utilized to construct combined models based on features derived from TIWI, T2WI, DTI, and MR elastography data. Statistical significance level was set at P < .05.
RESULTS
Demographics
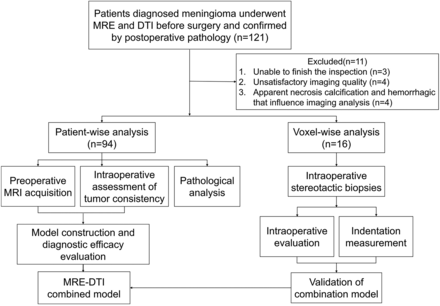
Fig 1 demonstrates the flow chart of the study. The patient characteristics are summarized in the Online Supplemental Data. Overall, 94 patients (25 men and 69 women) were enrolled in this study, for whom the average age was 54 ± 22 years. Most of the patients reported dizziness and headache as their primary symptom (38.3%), followed by physical health examination (30.9%), epilepsy (10.6%), and local neurologic deficits (11.7%). Pathologic diagnoses included 84 typical (grade 1) meningiomas and 10 atypical (grade 2) meningiomas. The typical meningiomas included the fibroblastic (34.0%), transitional (10.6%), meningothelial (38.3%), angiomatous (5.3%), and secretory (1.1%) tissue types. Moreover, total resection was achieved in most of the tumors.
Flow chart for the inclusion and exclusion criteria in the study cohort.
Radiologic and Intraoperative Findings
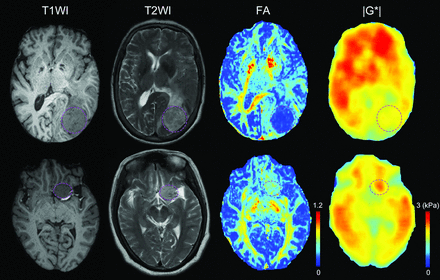
Representative images of T1WI, T2WI, FA map in DTI, and |G*| map in MR elastography are exhibited in Fig 2, including 1 patient with a soft meningioma and 1 with a firm meningioma. For all the images acquired, the average SI of delineated ROIs on FA map and |G*| map as well as SI ratio (tumor to cerebral cortex SI) on T1WI and T2WI were calculated and compared in “soft” and “firm” groups, as listed in the Table. The results showed that the |G*| between 2 groups was statistically significant (P < .0001), while there were no significant differences of T1WI, T2WI, and FA between 2 groups. Additionally, the correlation analysis demonstrated that |G*| was an independent variable from T1WI, T2WI, and FA (Online Supplemental Data).
A 66-year-old woman with soft meningioma (upper) in the left occipital lobe and a 58-year-old woman with hard meningioma (lower) in the left sphenoid ridge. The tumor ROIs were delineated with purple dashed lines in T1WI, T2WI, FA, and |G*| images from left to right.
Statistics of SI ratio (tumor to cerebral cortex SI) on T1WI and T2WI and ROI voxel values for FA, and |G*| images
Predictive Accuracy of Single Technique and Combined Model
The ROC curve was conducted based on mean SI calculated from ROI of tumor in each technique. The results of each individual technique and the combined technique are presented in Fig 3 The |G*| demonstrated the best diagnostic efficiency in singular modalities, with area under the curve (AUC) of 0.81 (95% CI: 0.699–0.930), followed by FA (AUC: 0.66, 95% CI: 0.531–0.785), and T2WI (AUC: 0.63, 95% CI: 0.507–0.768), while T1WI failed to predict tumor consistency (AUC: 0.51, 95% CI: 0.373–0.651). Furthermore, the use of combined modalities demonstrated significantly greater performance in determining meningioma consistency compared with single modalities. Notably, combined |G*|-FA exhibited the highest level of predictive accuracy with an AUC of 0.88 (95% CI: 0.784–0.971), closely tied with |G*|-T1WI (AUC: 0.82, 95% CI: 0.707–0.928) and |G*|-T2WI (AUC: 0.82, 95% CI: 0.706–0.925). This is consistent with the confusion metrics of each model, which yielded a similar outcome (Online Supplemental Data).
Comparison of diagnostic performance of modalities in evaluating meningioma consistency: (A) ROC curves based on T1WI, T2WI, FA, and |G*|; (B) ROC curves based on combinations of |G*|-T1WI, |G*|-T2WI, |G*|-FA.
Voxelwise Validation in Stereotactic Biopsies Using Indentation Measurement
Considering the heterogeneity of tumors, we performed 27 intratumoral stereotactic biopsies in 16 patients to further assess the reliability of the |G*|-FA combination by voxelwise analysis. The SI of ROI in each specimen's precise location, as recorded according to intranavigation, was calculated, and matched with the intraoperative records (Online Supplemental Data). Representative images of biopsy localization and H&E staining of specimens is displayed in the Online Supplemental Data. The voxelwise analysis showed that |G*|-FA combination was strongly correlated with intraoperative assessment of tumor consistency (P < .01, Fig 4A). Moreover, the indentation test was introduced as an objective criterion for assessing tumor consistency. The correlation analysis confirmed that the indentation measurement exhibited a substantial link with intraoperative evaluation (P < .0001, Fig 4B). Therefore, the indentation measurement was used to further validate the efficiency of the |G*|-FA combination. The combined technique showed a significant correlation with indentation results (P < .01, Fig 4C). Furthermore, a good correlation has been found in correlation analysis between indentation measurement and |G*| (P < .001, Online Supplemental Data).
Correlation analysis between (A) |G*|-FA and intraoperative assessment (r = 0.53, P = .004); (B) indentation measurement (G0), and intraoperative assessment (r = 0.72, P < .0001); and (C) |G*|-FA and indentation measurement (G0) (r = 0.53, P = .004).
Histologic Analysis to Investigate Underlying Mechanism
After performing an indentation test, histologic analysis was conducted on each specimen. Variability in tumor cellularity and fibrous content was observed among individual samples. Quantitative analysis of specimens indicated that the firm group possessed significantly higher cell density and larger fibrous content (P < .05, Online Supplemental Data). It indicated potential correlation between tumor consistency and its pathologic features, which could be identified by the presentation of the tumor on MRI.
DISCUSSION
In this study, we explored the diagnostic efficiency of combined MR modalities compared with single ones in predicting meningioma consistency. Indentation testing based on stereotactic biopsies further confirmed the generalizability of the patient-wise developed model in intratumoral voxelwise prediction. The results demonstrated the MR elastography–DTI model may be potentially practical for preoperatively assessing the tumor characteristics, which can help to optimize surgical strategies and predict operative risks, thereby minimizing the incidence of surgical complications and recurrence, and significantly improving the quality of surgery and patient prognosis.
MRI techniques have been used for predicting the consistency of intracranial meningiomas. Early studies found tumors showing hyperintense in T2-weighted images were more likely correlate with soft consistency (P < .01) but there was no correlation between T1WI and tumor consistency.10 Furthermore, the FA value from DTI was found to be a significant independent predictor of tumor consistency (P < .01).14 Consistent with these results, Romani et al16 concluded that isointense signal on MD maps (P < .01) and FA value >0.3 (P < .001) were significant indicators for predicting hard-consistency tumors. Nevertheless, there is no consensus on using MRI to determine tumor consistency.12,14,16⇓-18,35,37 Meanwhile, early MR elastography studies on brain tumors show the potential of using biomechanical properties to evaluate the consistency.27 In our study, it was confirmed that MR elastography exhibited better predictive accuracy than other MR modalities. Additionally, while MD showed a moderate diagnostic efficiency in diagnosing tumor consistency with AUC of 0.63, its combination with |G*| (AUC: 0.82, 95% CI: 0.7136–0.9283, Online Supplemental Data) did not demonstrate elevated efficacy compared with |G*|.
Recently, multimodal MRI has emerged as a potential tool for prediction of tumor consistency. A combined T1WI and T2WI for assessment showed a sensitivity of 90% and 56% (P < .001) for detecting soft and firm meningiomas, respectively.4 Furthermore, a 3D combination and display of multimodality images have been suggested to enhance the accuracy of interpreting cranial base tumors, potentially improving the safety of clinical procedures.40 Additionally, MRI radiomics is regarded as a potential tool for the surgical risk evaluation of meningiomas.41 Zhai et al42 demonstrated that radiomic analysis based on contrast-enhanced T1 weighted (T1CE) sequence of meningioma cases is a reliable predictor of tumor consistency. This suggests that radiomic analysis can be a valuable tool for the preoperative assessment of meningiomas. Despite these promising outcomes, the validation of the accuracy of such models in real practice has yet to be demonstrated. In this study, the combination of FA and |G*| can effectively predict the consistency of meningiomas with favorable accuracy preoperatively, with an AUC of 0.88, surpassing the performance of each technique alone. Meanwhile, the correlation coefficients presented by the voxelwise analysis, though not particularly high, demonstrated a significant correlation between the |G*|-FA combination and tumor consistency.
Given that heterogeneity of meningioma is an essential factor contributing to differences in surgical strategy and prognosis, it is crucial to precisely describe the consistency of tumor based on its exact intratumoral location.5,29 Hence, we conducted voxelwise analysis based on stereotactic biopsies to further validate the practical reliability of the combined model, where consistency of each tumor specimen was quantitatively measured by indentation test and calculated according to each technique. Our study revealed a strong correlation between the results of the combined model and tumor consistency, suggesting that the MR elastography–DTI model based on patient-wise analysis could be implemented for voxelwise intratumoral diagnosis.
As neuroimaging techniques continue to advance, offering more effective modalities for evaluating tumor consistency, the development of a precise and unbiased tumor consistency scoring system would greatly enhance our capacity to analyze and communicate surgical parameters regarding tumor consistency in a standardized manner. Previous studies have used grading scales of meningioma consistency according to the surgical instruments used and the working mode of the surgical instruments, or the differences in the ways of tumor resection. Zada et al43 proposed a 5-point scale grading system that relies on the ability of internal debulking and stiffness of the tumor capsule of the meningioma. It showed high agreement with a κ score of 0.87. Hughes et al29 graded meningioma consistency mainly based on which tool was utilized during tumor resection. The scale is straightforward but lacks quantitative criteria. Takamura et al44 defined intraoperative tumor consistency based on the Clarity Ultrasonic Surgical Aspirator amplitude applied for tumor removal. The grading criteria used in this study were based on the instrument used in the operation and its working mode, which were widely used by surgeons. Admittedly, diverse scales applied in research provides the possibility of discrepancies and interuser disagreements in determining tumor consistency because there is currently no uniform grading system for the consistency of meningiomas. Given that, we proposed the indentation measurement as quantitative technique for meningioma consistency evaluation. The correlation analysis between intraoperative evaluation and indentation measurements in our study demonstrated that objective assessments from experienced experts are consistent and reliable.
Studies have used novel techniques to assess the consistency of brain tumors. Della Pepa et al45 introduced intraoperative ultrasound elastography as a real-time imaging technique in the intraoperative prediction of meningioma consistency and brain-meningioma interface assessment. Due to the difficulty of obtaining images that match the standard radiologic planes and maintaining correct intraoperative spatial localization associated with it, the practicality of this technique remains to be demonstrated. Abramczyk et al46 utilized an indentation test for evaluating mechanical properties of high-grade medulloblastoma and identified significant variation in the mechanical properties of the tumor tissue. Additionally, a positive correlation was found between tissue consistency and pathologic grading of gliomas.47 In this study, we evaluated the accuracy of using indentation measurement as a quantitative determinant for meningioma consistency. The results indicated a substantial correlation between indentation results and intraoperative grading assessments, suggesting that indentation measurement can serve as an objective and reliable reference for estimating tumor consistency. According to the correlation analysis, the |G*|-FA combination exhibited a significant association with indentation outcomes.
Studies have reported a correlation between the consistency of meningiomas and their histopathology, with fibrous meningiomas more likely to be classified as stiff tumors.44 Recent studies demonstrated the impact of hypercellularity on shear stiffness in MR elastography, noting elevated shear stiffness in densely cellular meningothelial tumors.17 In our current study, we examined the variation of cellular density and fibrous content in soft and firm tumors. The results revealed that both factors were associated with tumor consistency, which may be reflected in imaging. Additionally, fibroblastic meningiomas exhibited higher FA values in DTI compared with other subtypes (Online Supplemental Data). It should be noted that the term “fibrous content,” which refers to the fibrous content of each tumor specimen as assessed by Masson staining, does not correspond to the pathologic subtype of meningioma known as “fibroblastic.” Fibroblastic meningiomas are characterized by spindle-shaped tumor cells, with narrow rod-shaped nuclei. These cells are embedded in abundant collagenous or reticulum background. Therefore, it does not mean that the fibrous content of fibroblastic meningioma is necessarily higher than that of other meningioma subtypes. The consistency of meningiomas may be related to the fibrous content, but not to the pathologic subtype. Nevertheless, future studies will investigate the underlying mechanobiologic mechanisms of meningioma consistency.
There are several limitations in this study. First, as a proof-of-concept study, only generalized autocalibrating partially parallel acquisition (GRAPPA) was used for the MR elastography scan with no further acceleration techniques. Therefore, the total scan time was ∼18 minutes. In fact, improved imaging protocol with accelerated sequence and reconstruction algorithm can significantly reduce the scan time. For example, if imaging the tumor area only with a single frequency, less than 4 minutes is needed. Second, only a limited number of tissue samples were obtained and validated by using the indentation measurement and histologic analysis. Third, T1 mapping or T2 mapping was not performed to obtain absolute values for quantification. Future work includes measurements with an enlarged sample size with ex vivo testing, and integration of novel modalities such as MR fingerprinting.
CONCLUSIONS
The MR elastography–DTI model proves to be a better predictor of meningioma consistency compared with other MR modalities (T1WI, T2WI, DTI) used alone or combined. In clinical practice, it could serve as an effective tool for preoperative assessment of tumor consistency, thus guiding the decision making of surgical strategy and assessment of surgical risk. With its help, maximum safety of resection as well as preservation of neurologic function may be achieved to reduce tumor recurrence and improve patients’ prognosis.
Footnotes
Yuting Bao, Suhao Qiu, and Zhenyu Li contributed equally to this work.
This study has received funding by National Natural Science Foundation of China (82272063, 82127801, 82227806, 32322042, 32271359), Shanghai Hospital Development Center (SHDC2020CR3073B), Shanghai Municipal Science and Technology Major Project (2018SHZDZX01), the Natural Science Foundation of Shanghai (22ZR1429600), the National Key R&D Program of China (2022YFB4702704, 2022YFB4702700), Science and Technology Commission of Shanghai Municipality (20DZ2220400), and Shanghai Municipal Science and Technology Major Project (2021SHZDZX) and ZJLab.
Disclosure forms provided by the authors are available with the full text and PDF of this article at www.ajnr.org.
References
- 1.
- 2.
- 3.
- 4.
- 5.
- 6.
- 7.
- 8.
- 9.
- 10.
- 11.
- 12.
- 13.
- 14.
- 15.
- 16.
- 17.
- 18.
- 19.
- 20.
- 21.
- 22.
- 23.
- 24.
- 25.
- 26.
- 27.
- 28.
- 29.
- 30.
- 31.
- 32.
- 33.
- 34.
- 35.
- 36.
- 37.
- 38.
- 39.
- 40.
- 41.
- 42.
- 43.
- 44.
- 45.
- 46.
- 47.
- Received April 11, 2024.
- Accepted after revision June 6, 2024.
- © 2024 by American Journal of Neuroradiology