Abstract
BACKGROUND AND PURPOSE: The impact of therapeutic embolization as a stand-alone treatment of head and neck paragangliomas considered surgically high-risk remains insufficiently understood. The aim of this study was to investigate the procedural risks and long-term volumetric development in head and neck paragangliomas with high surgical risk following therapeutic endovascular embolization as a stand-alone treatment.
MATERIALS AND METHODS: A retrospective database review of patients who underwent endovascular embolization as primary treatment for head and neck paragangliomas lacking appropriate curative treatment options at our institution (from January 2000 to February 2023) was conducted. Tumor volumetric analyses were performed before embolization and during follow-up. To assess the changes in tumor volume over time, the measurements were performed after embolization, first at 6 months and then on a yearly basis up to 6 years (mean follow-up time was 33.7 ± 24.4 months). Subgroup analyses were conducted for vagal and jugular/jugulotympanic paragangliomas.
RESULTS: A total of 32 head and neck paragangliomas in 28 patients (mean age, 56.1 years ± 16.5 [standard deviation]; 18 female) with therapeutic embolization as stand-alone treatment were evaluated, of which 11 were vagal paragangliomas, 15 jugular/jugulotympanic paragangliomas, and 6 carotid body tumors. After a mean follow-up duration of 33.7 ± 24.4 months, tumor control was achieved in 75%, with significant median tumor volume reduction at 6 months (P = .02, n = 21). Vagal paragangliomas responded the most to embolization with a significantly decreased median volume from 22.32 cm3 to 19.09 cm3 (P = .008, n = 8). Transient complications occurred in 3.4%.
CONCLUSIONS: Therapeutic embolization as a stand-alone treatment offers a low-risk control of tumor growth in surgically high-risk lesions, with a significant reduction in tumor volume after treatment. Among the different subtypes, vagal paragangliomas exhibited the strongest and longest regression of the tumor volume.
ABBREVIATIONS:
- HNPGL
- head and neck paraganglioma
- IQR
- interquartile range
- JTP
- jugular/jugulotympanic paraganglioma
- PVA
- polyvinyl alcohol
- SDH
- succinate dehydrogenase
- VP
- vagal paraganglioma
SUMMARY
PREVIOUS LITERATURE:
The impact and long-term effects of embolization of head and neck paragangliomas (HNPGLs) as an independent treatment option, not followed by resection or radiation therapy, remain insufficiently understood. The goal of such treatment is to induce devascularization-related long-term tumor control and symptom relief without aiming for cure. The relatively limited evidence suggests that embolization as a stand-alone treatment may be beneficial and considered as an alternative to radiation therapy in cases where surgery is not feasible or recommended.
KEY FINDINGS:
Our study demonstrates that particle embolization of HNPGLs, as an alone standing therapeutic choice, has low risks and effectively manages tumor growth in lesions deemed surgically high risk, achieving a notable decrease in tumor size. Among various subtypes, vagal paragangliomas (VPs) displayed the most pronounced and enduring reduction in tumor volume.
KNOWLEDGE ADVANCEMENT:
Our study advances the field by exclusively focusing on HNPGLs treated with therapeutic embolization as a stand-alone treatment and the performance of volumetric analysis to assess tumor growth. This study provides new insights into the management of HNPGLs, particularly for cases with a high surgical risk.
Head and neck paragangliomas (HNPGLs) are rare, benign neuroendocrine tumors that develop from paraganglia cells and are characterized by their slow growth and high vascularity, with only 6%–19% of all HNPGLs developing metastasis.1⇓⇓⇓-5 HNPGLs typically manifest between the ages of 40 and 70, with a higher prevalence among females, especially for noncarotid body tumors.5,6
HNPGLs are classified based on their anatomic locations. Carotid body tumors originate from paraganglia cells located at the carotid artery bifurcation, whereas vagal paragangliomas (VPs) arise from the inferior ganglion of the vagus nerve. Jugular paragangliomas arise from the jugular bulb near the skull base and tympanic paragangliomas in the middle ear along the Jacobson nerve. As these neoplasms originate from structures in proximity, they are often designated as jugulotympanic paragangliomas if they are large enough to be anatomically indistinguishable.7
Current management depends on symptoms, tumor size, location, growth rate, risk of malignancy, succinate dehydrogenase (SDH) and functional status, patient age, and overall health as well as expected treatment-related morbidity.1,5,6,8 Resection, with or without preoperative embolization, is the preferred treatment of choice for lesions with low surgical risk. Preoperative embolization is a well-established technique for tumor devascularization before surgery, reducing intraoperative bleeding risk, complications, and operation time.5,9,10 High surgical risk lesions, especially concerning the lower cranial nerve functionality, may be observed or treated with radiation therapy.5,6
Therapeutic embolization as a stand-alone treatment, not followed by resection or radiation therapy, may be beneficial and considered as an alternative to radiation therapy for cases with expected excessive surgical morbidity. However, its impact and long-term effects remain insufficiently understood. The goal of this approach is to induce devascularization-related long-term tumor control and symptom relief without aiming for cure. To establish itself as a beneficial option, this therapy should provide a more favorable outcome than the natural evolution, should have a similar effect as radiation therapy and low procedural risks, and may be repeatable over time.
Given the scarcity of available data, our objective was to examine both the procedural risks and the long-term evolution of paragangliomas managed along this concept at our institution.
MATERIALS AND METHODS
Ethics
This study was approved by the Ethical Committee for Clinical Research, Zurich, under the Business Administration System for Ethics Committees (BASEC) reference number 2022–00041.
Patient Selection
With a systematic retrospective database review, a total of 71 patients who underwent endovascular embolization for HNPGLs at our institution (from January 2000 to February 2023) were identified. The inclusion criteria comprised a diagnosis of nonfunctional HNPGL, no additional treatment besides embolization, availability of MR imaging scans before treatment, and at least 1 follow-up scan. Based on an interdisciplinary decision, therapeutic embolization as stand-alone treatment was considered in nonfunctional, progressive, and/or symptomatic HNPGL, as an alternative to radiation therapy, in cases with expected excessive surgical morbidity. HNPGLs were identified as carrying a high surgical risk due to the significant likelihood of causing permanent damage to the lower cranial nerves or critical vascular structures, such as the internal carotid artery. Patients with preoperative embolization were excluded from analysis. One additional patient with a jugulotympanic paraganglioma was considered an outlier due to an exceptionally rapid increase in volume and was secondarily excluded from the analyses.
Diagnostic Work-up
A standardized MR imaging protocol was performed in all cases, including axial T1- and T2-weighted, fat-saturated and T1-weighted, contrast-enhanced, fat-saturated Dixon sequences in 3 planes and a time-resolved, contrast-enhanced angiography of the supra-aortic vessels (Online Supplemental Data). The initial staging of HNPGLs included a comprehensive evaluation of tumor extension into bone and surrounding soft tissue by using a combination of anatomic and functional imaging approaches. MR imaging was used for assessing tumor location, size, and extension into soft tissues, bones, and neural structures. From a functional imaging perspective, a time-resolved, (4D) contrast-enhanced angiography of the head and neck arteries was conducted to visualize and identify arterial blood vessels feeding the tumor and to assess the kinetics of rapid wash-in and washout, which is characteristic of paraganglioma. In case of paragangliomas involving the skull base, high-resolution CT of temporal bones was used to assess bony invasion. Initial staging involved categorizing the anatomic extent of HNPGLs by using the Netterville-Glasscock classification for VPs,11 the Fisch and Mattox classification for jugular/jugulotympanic paragangliomas (JTPs),7 and the modified Shamblin classification for carotid body tumors, as suggested by Luna-Ortiz et al (Online Supplemental Data).12,13 SDH subtype characterization of the tumors was not routinely performed.
Endovascular Embolization
All embolization procedures were performed under general anesthesia. Initially, a complete diagnostic DSA work-up was performed to depict the arterial and venous supply, the angioarchitecture, and potentially dangerous anastomoses around the lesion. The embolization was subsequently performed with polyvinyl alcohol (PVA) (Boston Scientific) microparticles after superselective catheterization of the direct arterial feeders. The PVA particles were diluted in a 50%–50% saline and contrast solution and were injected while maintaining blood flow. The concept of particle embolization is based on persistent antegrade flow with the aim of deep penetration of smallest possible particles in the tumor vascular bed; pressure injections are avoided to reduce the penetration of particles into normal anatomic structures, such as the vascular supply of cranial nerves. As such, the size and concentration of particles was chosen by the treating neurointerventionalist in the range of 45–350 μ, based on the flow conditions in the feeder arteries and the presence of intratumoral shunts. In such conditions, liquid embolics (N-butyl cyanoacrylate or Onyx) were used to avoid particle migration into the veins. The postprocedure angiography was assessed based on residual tumor supply, and the level of the embolization was categorized by senior neurointerventionalists (T.S. and Z.K.) as partial (50%–90% of original supply), subtotal (90%–99%), or total (no residual supply).
Follow-up
Clinical and imaging follow-up was performed in a regular fashion to assess clinical evolution and tumor growth after embolization, first at 6 months and then on a yearly basis. The last MR imaging before the treatment was used to measure the baseline tumor volume, usually performed the day before or on the same day as the treatment (median, 1 day; interquartile range (IQR), 0.25). Tumor volume measurements were performed by manual segmentation by using Syngo.Via software (Syngo.Via Client 8.7, Siemens Healthineers) and were subsequently verified for accuracy. The verification process was conducted by neuroradiologists (R.L. and Z.K.). Radiographic progression was defined as a minimum of 15% volume increase at the last follow-up imaging; otherwise, tumor control was considered, as suggested in the literature.4,14,15
Statistical Analysis
Patient and tumor characteristics were described by using mean, standard deviation, median, IQR, and range for continuous variables and percentages for categoric features. Posttreatment tumor volumes were expressed relative to baseline volume, and mean relative tumor volumes were calculated for each follow-up time point. A cubic spline interpolation was used to visualize the trend of tumor volume changes over time. Data visualization was performed by using Matlab R2023a (MathWorks). Changes in the tumor volume before and after treatment were assessed for statistical significance by using a Wilcoxon signed-rank test for paired samples. Differences in the extent of volume change after subtotal, total, and partial embolization were evaluated by using the Wilcoxon rank-sum test (Mann-Whitney U test) for independent samples. P values < .05 were considered statistically significant. All statistical analyses were performed by using R software (R software version 4.3.1; R Foundation for Statistical Computing). Subgroup analyses were carried out for VP and JTP, with JTP encompassing jugular and jugulotympanic paragangliomas. No subgroup analyses for the carotid body tumors were performed due to a small number of tumors (n = 6).
RESULTS
Patient Characteristics
Baseline patient and tumor characteristics are summarized in the Table. Twenty-eight patients presented with 32 HNPGLs, 11 VPs (34.4%), 15 JTPs (46.9%), and 6 carotid body tumors (18.8%). Three patients (10.7%) had multiple HNPGLs: 1 patient with bilateral VP, 1 patient with bilateral carotid body tumors and a jugular paraganglioma, and 1 patient with both a JTP and VP. The mean age at treatment was 56.1 years ± 16.5 (mean ± standard deviation), and most patients were female (64.3%, n = 18). The mean follow-up duration for radiographic imaging was 33.7 months ± 24.4, and mean baseline tumor volume was 20.51 cm3 ± 18.94.
Patient and tumor characteristicsa
Embolization Results
Complete devascularization, achieved by total embolization of all visualized arterial feeders, was achieved in 7 (21.9%) cases, subtotal devascularization in 20 (62.5%), and partial devascularization in 5 (15.6%) (Fig 1). Among the HNPGLs with complete devascularization, 6 had 3 or fewer arterial feeders visualized, whereas only 1 had more than 3. In cases of subtotal devascularization, 7 had 3 or fewer arterial feeders, whereas 13 had more than 3. All HNPGLs with partial devascularization had more than 3 arterial feeders visualized. One patient with a jugular paraganglioma experienced a postinterventional reduction of visual acuity due to ipsilateral retinal artery branch occlusion, completely resolved over 6 months. No other patient developed new neurologic deficits after embolization, meaning an embolization-related complication of 3.4%, without permanent morbidity.
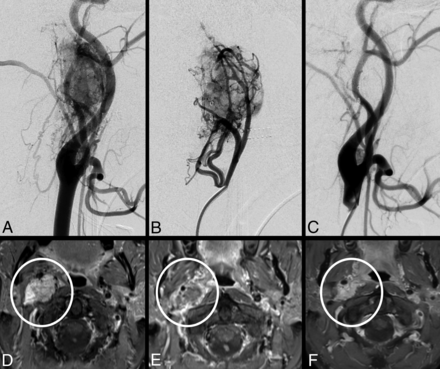
Vagal paraganglioma on the right side, lateral-view DSA of the right common carotid artery (A) and superselective injection of the direct supply from the ascending pharyngeal artery before embolization (B). After particle embolization, the complete devascularization of the tumor is shown (C). T1-weighted, fat-saturated, gadolinium-enhanced axial MR imaging of the same vagal paraganglioma before (D), 1 day after (E), and 2 years after (F) therapeutic embolization as stand-alone treatment (inside white circle). Note the immediate tumor shrinkage and lack of contrast enhancement at 24 hours. After 2 years (F), the tumor is still significantly smaller as compared with pre- and immediately postembolization (D and E), despite it showing recurrent contrast enhancement.
Volumetric Assessment of the Entire Cohort of HNPGL.
Volumetric data were available for n = 21 HNPGLs at 6 months (mean ± standard deviation, 5.8 ± 1.9), n = 17 HNPGLs at 12 months (14 ± 3.8), n = 14 HNPGLs at 24 months (23.8 ± 3.7), n = 10 HNPGLs at 36 months (33.3 ± 4.4), n = 9 HNPGLs at 48 months (47.7 ± 3.9), n = 6 HNPGLs at 60 months (56.2 ± 2.9), and n = 6 HNPGLs at 72 months (71.8 ± 2.9). In total, 115 MR imaging studies were analyzed with a mean of 3.6 ± 1.7 studies per paraganglioma (Fig 2).
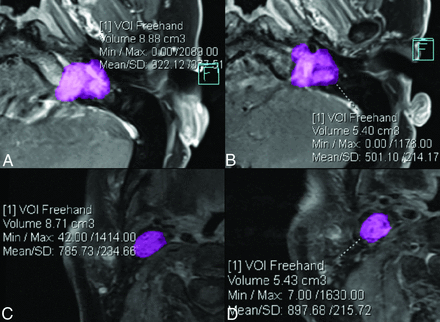
Axial T1-weighted, fat-saturated, gadolinium-enhanced MR imaging of a left jugular paraganglioma pretreatment (A) and at 5-month follow-up (B) and of a right vagal paraganglioma pretreatment (C) and at 27-month follow-up (D). The tumor volume measurements are displayed in magenta.
Following embolization and compared with the pretreatment status, the mean relative tumor volume was reduced to 92.1% at 6 months, 92.9% at 12 months, 90.9% at 24 months, 98.6% at 36 months, 109.4% at 48 months, 105.5% at 60 months, and 93.4% at 72 months. Figure 3 (solid line) further illustrates this trend of tumor volume change over time. It shows that a minimal mean relative volume was achieved after 2 years, and the time to return to the baseline was approximately 3 years.
Time-dependent change of mean tumor volume following embolization as a stand-alone treatment. Mean tumor volume is plotted relative to baseline volume for all 32 head and neck paragangliomas (total; solid line), for 11 vagal paragangliomas (VP; dotted line), and for 15 jugular/jugulotympanic paragangliomas (JTP; dash-dotted line). The baseline volume of 100% is shown by the dashed straight line.
A significant reduction in tumor volume was observed after 6 months (P = .02, n = 21). The median baseline volume decreased from 13.98 cm3 (IQR, 22.77 cm3; range, 2.49–89.08 cm3) to 12.52 cm3 (IQR, 20.04 cm3; range, 1.70–88.46 cm3) after 6 months, yielding a 10.4% decrease. At the other follow-up time points, no further significant reduction or increase compared with baseline volume was observed.
Volumetric Assessment for VPs and JTPs.
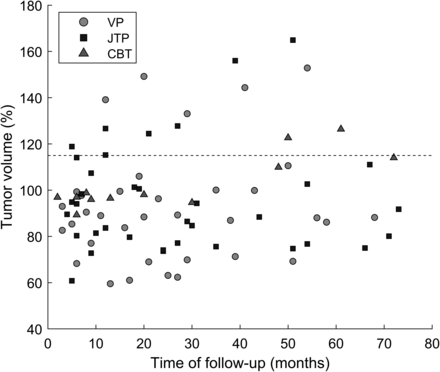
The mean relative tumor volume for VPs after embolization was reduced to 86.7% at 6 months (n = 8), 85.9% at 12 months (n = 7), 90.8% at 24 months (n = 7), 96.1% at 36 months (n = 5), 106.0% at 48 months (n = 4), and 109.0% at 60 months (n = 3). The tumor volume was significantly reduced at 6 months (P = .008, n = 8). The median pretreatment volume was 22.32 cm3 (IQR, 31.24 cm3; range, 2.49–89.08 cm3), whereas the median volume at 6 months was 19.09 cm3 (IQR, 30.18 cm3; range, 1.70–88.46 cm3), resulting in a 14.5% decrease. For JTPs, the mean relative tumor volume following embolization was 95.4% at 6 months (n = 9), 98.2% at 12 months (n = 8), 89.9% at 24 months (n = 6), 102.7% at 36 months (n = 4), 109.4% at 48 months (n = 3), 89.7% at 60 months (n = 2), and 89.5% at 72 months (n = 4). These trends are illustrated in Fig 3 with a dotted curve for VPs and a dash-dotted curve for JTPs. It shows that mean relative tumor volume reached a minimum at 9 months with a reduction of 14.6% for VPs and at 24 months with a reduction of 10.1% for JTPs. The time to return to the baseline was approximately 42 months for VPs and 34 months for JTPs. The individual volume measurements are shown in Fig 4. Online Supplemental Data provide additional details on the volumetric response of HNPGLs to endovascular embolization.
All measurements are expressed relative to the baseline volume. The carotid body tumors (CBT) are represented as triangles, the vagal paragangliomas (VP) as circles, and the jugular/jugulotympanic paragangliomas (JTP) as squares. The dashed line indicates a volume change of +15%, the definition of radiographic progression.
Tumor Control
According to the definition of radiographic progression, at the most recent posttreatment MR imaging, 24 HNPGLs had a reduction in volume or remained unchanged, whereas 8 HNPGLs experienced a radiographic progression. Therefore, tumor control was achieved in 75% after a mean follow-up duration of 33.7 ± 24.4 months. Out of 32 HNPGLs, 8 decreased in volume by more than 15%, where 4 of these HNPGLs underwent total and 4 subtotal embolization. Of the 8 HNPGLs with more than 15% increase in volume, 4 had subtotal and 4 had partial embolization. Total and subtotal embolizations were associated with significantly stronger volume reductions compared with partial embolization (P = .003 and P = .04, respectively). The median changes in relative volume were −19.9% (IQR, 26.3%) for total and −3.3% (IQR, 24.1%) for subtotal, as opposed to +18.9% (IQR, 11.2%) for partial embolization.
DISCUSSION
In this study, we analyzed the growth rate of HNPGLs after therapeutic embolization as a stand-alone treatment for tumors bearing excessive surgical risk. The main results are a comparably high rate of tumor control and a very low procedural risk. Our study showcases the effectiveness of therapeutic embolization as a stand-alone treatment for HNPGLs with excessive surgical risk by successfully reducing tumor size in the short term and controlling tumor growth over an extended period, particularly when complete devascularization is accomplished. Given the low risk associated with particle embolization and the procedure’s repeatability, therapeutic embolization as stand-alone treatment presents a viable long-term alternative for patients at high surgical risk.
Embolization versus Natural Evolution
Given the very slow growth rate of untreated HNPGLs, the tumor control by therapeutic embolization has to show clinical relevance. Jansen et al3 (2000) found in their study of 48 untreated HNPGLs in 26 patients a median growth rate of 0.83 mm/year, with a tumor doubling time of 10.15 years. Langerman et al2 (2012) reported that, during a mean follow-up of 5 years, 42% remained stable, 20% diminished, and 38% of the 45 HNPGLs in their study exhibited growth with a mean growth rate of 0.2 cm/year.
Tamaki et al4 (2022) observed a volume increase >15% in 43.6% of 39 HNPGLs after a median follow-up time of 1.95 years. They also reported a median tumor doubling time of 5.67 years in the growing HNPGLs. In contrast to the natural history data, in our study, only 8 of 32 HNPGLs (25%) experienced a volume increase >15% after a longer follow-up duration (mean follow-up duration of 33.7 months ± 24.4), demonstrating that embolization as a stand-alone treatment is able to achieve tumor control at a higher rate compared with the natural evolution, especially when a total devascularization can be achieved.
Embolization versus Radiation Therapy
As an alternative to surgical resection, radiation therapy is most often proposed. It can slow tumor growth, and it has a reduced risk of morbidity and lower cranial nerve deficits compared with surgery.5,16,17 Fatima et al18 (2021) conducted a meta-analysis comprising 1144 HNPGLs, revealing a local control rate of 94.2% after adjuvant or primary stereotactic radiosurgery with a median follow-up of 44 months. Radiologic outcome showed stable disease in 48.7%, progressive disease in 5.8%, and a tumor shrinkage in 45.5%.18 Anderson et al19 (2020) assessed the tumor size reduction after intensity-modulated radiation therapy and stereotactic radiosurgery. After a median follow-up of 4.16 years, 26 of 30 tumors decreased in volume whereas 4 increased.19 Dharnipragada et al17 (2023) reported a 96.3% tumor control rate in a meta-analysis of 153 JTPs treated with stereotactic radiosurgery. Complication rates ranged from 5.5% to 15% depending on the study.17,19⇓-21 Our results showed comparable results in tumor growth control and complications in patients with high surgical risk, with tumor control rate of 75% after a mean follow-up duration of 33.7 months ± 24.4 and a low rate of transient morbidity of 3.4%.
The Concept of Particle Embolization
The goal of embolization is to selectively obliterate the abnormal microvascular structure of a lesion while preserving normal blood supply to surrounding tissues. Preoperative embolization is used to devascularize tumors before surgery to reduce blood loss, decrease tumor volume, minimize surgical complications, and prevent recurrence by facilitating complete resection.5,9,10 Though sometimes debated, the positive effects and low complications rates of preoperative embolization have been relatively well addressed in the literature. The complications rate of our series with particle embolization (3.4% of transient complications) reflects the results of published data on preoperative embolization.22⇓⇓⇓-26
However, in contrast to preoperative devascularization aiming to facilitate resection and improve results, the primary objective of embolization as a stand-alone treatment is to achieve long-term tumor growth control by inducing tumor shrinkage and symptom relief to improve the quality of life. However, it is crucial to acknowledge that the effectiveness of therapeutic embolization as a stand-alone treatment can be constrained by the tumor’s potential for growth, which may arise from revascularization over time and the presence of small feeding vessels that remain undetected or unreachable during endovascular treatment, thus evading total occlusion.27,28 Our choice for particle embolic agents was justified by the lower rate of major complication compared with liquid embolic agents, especially what concerns iatrogenic cerebral embolizations and cranial nerve palsies.22,29 Furthermore, particle embolization allows repeatability of the treatment if necessary. Along the concept of intratumoral devascularization and by not occluding them during the treatment, the major feeding arteries remain repeatedly accessible in the future.
There are limited reports on therapeutic embolization as a stand-alone treatment for HNPGLs. In a study by Tasar et al28 (2004), 17 patients undergoing either palliative or preoperative embolization by coils or combination of coils and PVA were monitored over a follow-up duration of 4 to 15 months. Out of these, 11 patients underwent embolization as a stand-alone treatment, and no tumor growth was observed during follow-up. Michelozzi et al29 (2016) utilized embolization with Onyx in 19 patients alone or combined with radiation therapy (5 patients) and tympanic surgery postembolization (3 patients). In subanalysis for the JTPs, 12 tumors out of 16 remained stable (median follow-up 29 months), whereas 1 exhibited tumor progression. Tumor control was achieved in 7 out of 10 patients (mean follow-up 45.7 months) who only underwent embolization. A further study followed 2 patients who received therapeutic embolization with isobutyl 2-cyanoacrylate; they showed neither progression nor recurrence during a follow-up period of 32 and 38 months.30 The growth control we achieved with particle embolization resembled these reported findings, however with a lower complication rate.
Nevertheless, there are limitations of our study. Given its retrospective nature, the timing of imaging studies was not strictly standardized, and we were limited to follow-up time points where enough volumetric data were available for the analysis of volume evolution. Further, fewer imaging studies were available for later time points, which reduced the power of the analysis toward the end of follow-up and made it difficult to achieve statistical significance. Additionally, the characterization of SDH subtypes for the tumors was not conducted in this series, and no subgroup analyses were performed for carotid body tumors due to their limited number.
CONCLUSIONS
Particle embolization of HNPGLs, as a stand-alone therapeutic choice, has low procedural risks and effectively manages tumor growth in lesions deemed surgically high risk, achieving a notable decrease in tumor size in the initial years posttreatment, especially if a subtotal or total devascularization was achieved. Among various subtypes, VPs displayed the most pronounced and enduring reduction in tumor volume. Based on these results, particle embolization as a stand-alone treatment may be considered as an alternative therapeutic option in the management of HNPGLs.
Footnotes
Disclosure forms provided by the authors are available with the full text and PDF of this article at www.ajnr.org.
References
- 1.
- 2.
- 3.
- 4.
- 5.
- 6.
- 7.
- 8.
- 9.
- 10.
- 11.
- 12.
- 13.
- 14.
- 15.
- 16.
- 17.
- 18.
- 19.
- 20.
- 21.
- 22.
- 23.
- 24.
- 25.
- 26.
- 27.
- 28.
- 29.
- 30.
- Received November 21, 2023.
- Accepted after revision April 30, 2024.
- © 2024 by American Journal of Neuroradiology