Abstract
BACKGROUND AND PURPOSE: The susceptibility vessel sign, a hypointense signal on MR T2-weighted gradient-recalled echo images, is associated with erythrocyte-predominant thrombi, which are often present in cardioembolism. In contrast, cancer-associated hypercoagulability (CAH)-related stroke, which is presumably caused by fibrin-predominant thrombi, is associated with the absence of the susceptibility vessel sign. We hypothesized that the prevalence of the susceptibility vessel sign may be helpful in distinguishing CAH-related stroke from cardioembolism. This study attempted to validate this hypothesis and investigated the usefulness of the susceptibility vessel sign in differentiating CAH-related stroke from cardioembolism.
MATERIALS AND METHODS: We retrospectively studied patients with both CAH-related stroke (CAH group) and cardioembolism (cardioembolism group) who had major cerebral artery occlusion on MRA that was performed within 6 hours of stroke onset. All patients visited our department from 2015 to 2021. CAH-related stroke was defined as the following: 1) complication of active cancer, 2) pretreatment D-dimer value of >3 μg/mL, 3) multiple vascular territory infarctions, and 4) lack of any other specifically identified causes of stroke. We compared susceptibility vessel sign positivity rates within each group. Multivariable logistic regression analysis was used to assess the association between the absence of the susceptibility vessel sign and CAH-related stroke.
RESULTS: Of 691 patients with CAH-related stroke or cardioembolism, major cerebral artery occlusion was observed in 10 patients in the CAH group and 198 patients in the cardioembolism group. The absence of the susceptibility vessel sign was identified in 55 of 208 patients and was significantly more frequent in the CAH group versus the cardioembolism group (90% versus 24%, P < .05). For predicting CAH-related stroke, the absence of the susceptibility vessel sign demonstrated a sensitivity of 90% (95% CI, 59%–99%), specificity of 78% (95% CI, 71%–83%), a positive predictive value of 18% (95% CI, 10–31), a negative predictive value of 99% (95% CI, 96%–99%), and a likelihood ratio of 4.06. Multivariable logistic regression analysis revealed that the absence of the susceptibility vessel sign was independently associated with CAH-related stroke (OR, 43; 95% CI, 6.8–863; P < .01).
CONCLUSIONS: The absence of the susceptibility vessel sign was more frequent in CAH-related stroke than in cardioembolism. These findings could potentially be helpful for clinical management and differentiating cardioembolism and CAH-related stroke.
ABBREVIATIONS:
- CAH
- cancer-associated hypercoagulability
- CE
- cardioembolism
- GRE
- gradient-recalled echo
- SVS
- susceptibility vessel sign
SUMMARY
PREVIOUS LITERATURE:
Susceptibility vessel sign (SVS) has been shown to be associated with the component of the thrombi. The SVS positivity rate is high in cardioembolism (CE)due to the erythrocyte-predominant thrombi, while in atherothrombotic brain infarction, the rate is low due to the fibrin-predominant thrombi. Moreover, it has been reported that a higher proportion of stroke patients with active cancer exhibit the absence of SVS as compared with those without. However, there are no reports focusing on cancer associated hypercoagulability (CAH)-related stroke in patients with active cancer.
KEY FINDINGS:
The absence of SVS was more frequent in CAH-related stroke compared with that for CE and it was independently associated with the CAH-related stroke. When differentiating between these two types of strokes, the absence of SVS indicates there is a higher likelihood of CAH-related stroke.
KNOWLEDGE ADVANCEMENT:
The difference in thrombopathology may attribute to the difference in the frequency of SVS in CE and CAH-related stroke. The absence of SVS is valuable in distinguishing between CE and CAH-related stroke in embolic stroke of undetermined source and may serve as a guide in making clinical decisions in these cases.
Patients with cancer face an elevated risk of ischemic stroke, with cancer-associated hypercoagulability (CAH) being the primary mechanism contributing to this risk.1⇓-3 CAH-related stroke is characterized by the presence of high D-dimer levels and multiple vascular territory infarctions.4⇓-6 In some cases, patients with CAH-related stroke experience major cerebral artery occlusion and have MR imaging findings similar to those observed for cardioembolism (CE).7⇓-9
CAH-related stroke and CE both require antithrombotic therapy to prevent recurrence, but the choice of the agent is specific for each mechanism. Typically, direct oral anticoagulants or warfarin is used in CE, while subcutaneous heparin is preferred in CAH-related stroke.2,10 Thus, it is crucial to distinguish between CAH-related stroke and CE to prevent stroke recurrence.
Furthermore, CE and CAH-related stroke contribute to the etiology of embolic stroke of undetermined source. Given the differing clinical management strategies for suspected CE and CAH-related stroke with the etiology of embolic stroke of undetermined source, the differentiation between the 2 conditions is crucial in determining the clinical management strategies.
The susceptibility vessel sign (SVS), which is detected as a hypointense signal on the T2* gradient-recalled echo (GRE) images, is associated with a high proportion of deoxyhemoglobin within the thrombus. Erythrocyte-rich thrombi, which are typically seen in CE, are likely to show the SVS, while fibrin-predominant thrombi, which are typically found in atherothrombotic brain infarction, are less likely to show this. It has been previously reported that the SVS can be useful in helping to distinguish atherothrombotic brain infarction from CE.11⇓⇓⇓-15 Although the thrombus found in CAH-related stroke has also been reported to be fibrin-predominant,8,16,17 the SVS has yet to be fully investigated in patients with CAH-related stroke; thus, the diagnostic role of the SVS in CAH-related stroke remains unclear. The purpose of this study was to investigate the usefulness of the SVS in distinguishing CAH-related stroke from CE.
MATERIALS AND METHODS
Design
This study was a retrospective observational study of patients who were diagnosed with ischemic stroke at a tertiary stroke center (Kyoto Second Red Cross Hospital, Kyoto, Japan). The need to obtain informed consent for participation was waived due to the retrospective design and the minimal risk to patients.
Study Patients
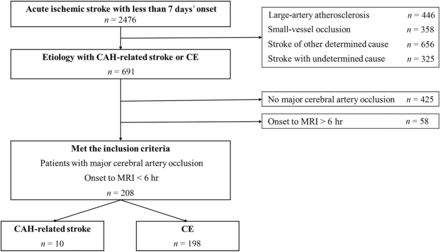
Of the 2476 patients with acute ischemic stroke who were admitted to the Kyoto Second Red Cross Hospital within 7 days after stroke onset between April 2015 and March 2021, we enrolled patients with both CAH-related stroke and CE with major cerebral artery occlusion. Acute ischemic stroke was defined as any new neurologic symptom with acute ischemic lesions confirmed by DWI. CAH-related stroke was defined using the following criteria: 1) complication of active cancer, 2) pretreatment D-dimer value of >3 μg/mL, 3) multiple vascular territory infarctions, and 4) lack of any other specifically identified causes of stroke.18 CE was classified according to the Trial of Org 10172 in Acute Stroke Treatment (TOAST) criteria.19 Major cerebral artery occlusion was defined as carotid T occlusion (occlusion of the carotid artery, middle and anterior cerebral artery), M1 MCA occlusion, or M2 MCA occlusion. We excluded patients who did not undergo MR imaging within 6 hours of onset. Active cancer was defined as a diagnosis or treatment for any cancer within 6 months before ischemic stroke onset or known recurrent cancer or metastatic disease. Patients with focal nonmelanoma skin cancer and those treated with prophylactic hormone therapy for prior breast cancer were classified as not having active cancer.20
Measurements
Using previous medical records, we collected data regarding age, sex, cardioembolic source, type of cancer, time from onset to MR imaging, pretreatment D-dimer levels, DWI findings, and vascular imaging. The 3-territory sign was defined as the presence of lesions in the 3 vascular territories of the bilateral anterior and posterior circulation.21
Outcomes
The primary outcome was the positivity of the SVS. The SVS was defined as a hypointense signal on T2* GRE at a corresponding symptomatic occlusive vessel, for which the signal exceeded the estimated diameter of the artery (Fig 1).22 The SVS was assessed by 2 independent stroke-specialized neurologists (D.F. and J.F.). When the judgment of the 2 neurologists was inconsistent, a decision was made by discussion without the consideration of any information other than the T2* GRE images.
Assessment of the SVS. Complete occlusion of the right MCA on arterial TOF (A), with the presence of the SVS on the T2* GRE (B). Complete occlusion of the right MCA on arterial TOF (C), with the absence of the SVS on the T2* GRE (D). The arrowheads indicate the part of the vessel occlusion on the TOF and T2* GRE images.
MR Imaging Protocol
We used 2 MR imaging models: Ingenia 1.5T (Philips Healthcare) and Magnetom Avanto 1.5T (Siemens). The T2* GRE sequence was examined using the following parameters: TR/TE = 459/18 ms, flip angle = 20°, section thickness = 6 mm, section gap = 0.6 mm, acquisition time = 80 seconds, matrix = 208 × 209, and field of view = 220 mm.
Statistical Analysis
The Fisher exact test and the Mann-Whitney U test were used as appropriate to compare the clinical characteristics between the CAH and the CE groups. The κ coefficient was used to determine the interobserver agreement for the SVS on T2* GRE. Multivariable logistic regression analysis was used to investigate the association between the absence of SVS and CAH-related stroke. The level of significance was considered .05 in all tests. All statistical analyses were performed using GraphPad Prism, Version 9.4.1 (GraphPad Software).
RESULTS
A flow diagram of the patient selection is presented in Fig 2. The final cohort consisted of 208 patients with acute ischemic stroke with major cerebral artery occlusion, which included 10 in the CAH and 198 in the CE groups. The most prevalent cancer types were lung cancer (3 cases) and pancreatic cancer (3 cases). For CE, embolic sources included atrial fibrillation in 186 cases, symptomatic congestive heart failure in 5 cases, paradoxical embolism in 4 cases, and recent myocardial infarction, calcified aortic stenosis, and mechanical valve in 1 case each. Table 1 presents the baseline characteristics.
Study flow chart for the inclusion of subjects.
Baseline characteristicsa
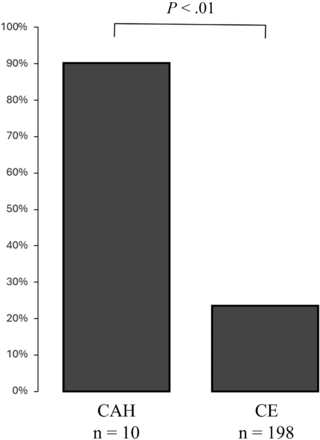
The SVS was absent in 55 patients (26%), with 9 cases in the CAH group and 46 cases in the CE group. The absence of the SVS was more frequent in the CAH-versus-CE groups (P < .01) (Fig 3). The absence of the SVS was observed in 22% for the ICA, 11% for M1, and 29% for M2. The κ coefficients for interobserver agreement of the SVS by the 2 examiners were 0.87 and 0.98, while the κ coefficient was 0.92 for interobserver agreement of the SVS. The disagreement rate between the 2 examiners was 9%.
Proportion of the absence of the SVS in each group.
To assess the performance of the absence of the SVS with regard to differentiating CAH-related stroke from CE, we used multivariable logistic regression analysis to identify the association factor for the CAH-related stroke. Results revealed that the absence of the SVS was independently associated with CAH-related stroke (OR, 43; 95% CI, 6.8–860; P < .01) (Table 2). The absence of the SVS demonstrated a sensitivity of 90% (95% CI, 59%–99%), specificity of 78% (95% CI, 71%–83%), a positive predictive value of 18% (95% CI, 10%–31%), a negative predictive value of 99% (95% CI, 96%–99%), and a likelihood ratio of 4.06 for predicting CAH-related stroke. The presence of the SVS demonstrated a sensitivity of 78% (95% CI, 71%–83%), specificity of 90% (95% CI, 59%–99%), a positive predictive value of 99% (95% CI, 96%–99%), a negative predictive value of 18% (95% CI, 9%–30%), and a likelihood ratio of 7.78 for predicting CE.
Multivariable logistic regression analysis for the absence of the SVS
DISCUSSION
The present results show that the absence of the SVS was more frequent in CAH-related stroke compared with CE. When one differentiates these 2 types of strokes, the absence of the SVS indicates that there is a higher likelihood of CAH-related stroke.
The high proportion of CAH-related stroke with the absence of the SVS may be attributed to the fibrin-predominant thrombi that were present. The SVS has been shown to be associated with the presence of deoxyhemoglobin, methemoglobin, and hemosiderin within the erythrocytes of the thrombi.11,23 In fact, the SVS positivity rate is high (56%–89%) in CE due to the erythrocyte-predominant thrombi, while in atherothrombotic brain infarction, the rate is low (19%–53%) due to the fibrin-predominant thrombi.12,14,24,25 Similarly, it has been reported that a higher proportion of patients with stroke with active cancer show the absence of the SVS compared with those without active cancer.20 This finding might be because fibrin-predominant thrombi are more frequently observed in patients with stroke with active cancer.26 Fibrin-predominant thrombi are more frequently observed in CAH-related stroke, which is the main subtype of stroke in patients with active cancer, compared with thrombi found in CE or atherothrombotic brain infarction.27 In the present study, we selected study subjects with CAH-related stroke from any of the stroke subtypes in patients with active cancer. Thus, the proportion of CAH-related stroke with the absence of the SVS was markedly high at 90%.
In recent years, mechanical thrombectomy has provided insight into the characteristics of retrieved thrombus. Case reports have shown the presence of fibrin-predominant thrombus pathology in CAH-related stroke.8,16,17 Thrombus formation in CAH-related stroke involves multiple mechanisms leading to hypercoagulability. First, when the vascular endothelium is damaged by high flow or inflammatory cytokines produced by cancer cells, platelets will then adhere to the exposed area of the endothelium. Subsequently, platelets aggregate through an interaction with the activated Von Willebrand factor. Cancer cells cause hypercoagulability through mechanisms such as activating the coagulation cascade with tissue factor, directly activating prothrombin with mucin, and directly activating factor X with cysteine protease. The hypercoagulability leads to the formation of a fibrin-predominant thrombus under high-flow arterial conditions, which then leads to less entrapment of the red blood cells.28
The findings of the present study have the potential for clinical impact in being able to distinguish between CE and CAH-related stroke within the context of embolic stroke of undetermined source. Embolic stroke of undetermined source represents a subset of cryptogenic strokes and accounts for approximately 25% of all ischemic strokes. The causes of embolic stroke of undetermined source include subclinical paroxysmal atrial fibrillation, aortogenic embolism, paradoxical embolism, and cancer-associated stroke.29 While these etiologies all have similar features such as embolic stroke, microvascular invasions, and elevated D-dimer levels, the aortogenic embolism and paradoxical embolism can be diagnosed through additional modalities such as contrast-enhanced angiography or transesophageal echocardiography. As in the present study, although CE is characterized by elderly age compared with CAH-related stroke, the differentiation between CE and CAH-related stroke remains challenging. When one tries to identify the specific causes, such as subclinical paroxysmal atrial fibrillation, the underlying malignancy remains elusive in these cases. In such cases, the absence of the SVS may serve as a guide in making clinical decisions. In summary, the SVS serves as a guide for clinical management strategies. These strategies may involve repeat Holter ECG monitoring or the use of an implantable loop recorder. Conversely, if the SVS is absent, vigilant monitoring will be necessary to detect these malignancies. This includes further investigations and early-stage re-examination of the cancer using CT scans or other diagnostic modalities.
However, there were several limitations for the present study. First, this was a single-center, retrospective study; thus, a selection bias may exist. Also, given the small number of study patients and low incidence of CAH-related stroke, the sample size was significantly unbalanced, reducing the power of statistical analysis. Thus, further studies with larger numbers of cases and multicenter studies are needed. Second, there were some disagreements with the SVS assessment between the 2 examiners. However, the interobserver agreement was high enough to be considered satisfactory. Third, the section thickness and section gap of the T2* GRE at our center were 6/0.6 mm, which is rather thick for assessing cerebral vessels; thus, these features may have affected the SVS evaluation. Moreover, because the SVS evaluation did not use SWI, which is more sensitive than T2* GRE, it is possible that the absence of the SVS may have been overestimated. Fourth, because the appearance and quality of T2*GRE can vary, the present findings may not be fully generalizable. Finally, the thrombus pathology was not assessed. Nevertheless, because in actual clinical practice the thrombopathology is not always confirmed, the results of the current study may be clinically valuable regarding future diagnostic and management strategies.
CONCLUSIONS
The absence of the SVS was more frequent in CAH-related stroke compared with CE. Thus, the absence of the SVS might be an important factor for the clinical management and the ability to differentiate CE and CAH-related stroke.
Footnotes
Disclosure forms provided by the authors are available with the full text and PDF of this article at www.ajnr.org.
References
- 1.
- 2.
- 3.
- 4.
- 5.
- 6.
- 7.
- 8.
- 9.
- 10.
- 11.
- 12.
- 13.
- 14.
- 15.
- 16.
- 17.
- 18.
- 19.
- 20.
- 21.
- 22.
- 23.
- 24.
- 25.
- 26.
- 27.
- 28.
- 29.
- Received February 16, 2024.
- Accepted after revision May 27, 2024.
- © 2024 by American Journal of Neuroradiology