SUMMARY:
Given the recent advances in molecular pathogenesis of tumors, with better correlation with tumor behavior and prognosis, major changes were made to the new 2021 World Health Organization (WHO) classification of CNS tumors, including updated criteria for diagnosis of glioblastoma (GBM). Diagnosis of GBM now requires absence of isocitrate dehydrogenase and histone 3 mutations (IDH-wild-type and H3-wild-type) as the basic cornerstone, with elimination of the IDH-mutant category. The requirements for diagnosis were conventionally histopathological, based on the presence of pathognomonic features such as microvascular proliferation and necrosis. However, even if these histologic features are absent, many lower-grade (WHO grade 2/3) diffuse astrocytic gliomas behave clinically similar to GBM (grade 4). The 2021 WHO classification introduced new molecular criteria that can be used to upgrade the diagnosis of such histologically lower-grade, IDH-wild-type, astrocytomas to GBM. The 3 molecular criteria include: concurrent gain of whole chromosome 7 and loss of whole chromosome 10 (+7/–10); telomerase reverse transcriptase promoter mutation; and epidermal growth factor receptor amplification. Given these changes, it is now strongly recommended to have molecular analysis of WHO grade 2/3 diffuse astrocytic, IDH-wild-type, gliomas in adult patients, as identification of any of the above mutations allows for upgrading the tumor to WHO grade 4 (“molecular GBM”) with important prognostic implications. Despite an early stage, there is active ongoing research on the unique MR imaging features of molecular GBM. This paper highlights the differences between “molecular” and “histopathological” GBM, with the aim of providing a basic understanding about these changes.
ABBREVIATIONS:
- cIMPACT-NOW
- Consortium to Inform Molecular and Practical Approaches to CNS Tumor Taxonomy-Not Official WHO
- CNS5
- 2021 WHO classification of CNS tumors
- EGFR
- epidermal growth factor receptor
- GBM
- glioblastoma
- GFAP
- glial fibrillary acidic protein
- GSEA
- gene set enrichment analysis
- IDH
- isocitrate dehydrogenase
- MGMT
- methylguanine-DNA methyltransferase
- MVP
- microvascular proliferation
- NGS
- next-generation sequencing
- TERT
- telomerase reverse transcriptase
- TMZ
- temozolomide
- WHO
- World Health Organization
Glioblastoma (GBM) is the most common and aggressive primary malignant brain neoplasm worldwide, accounting for approximately 15% of all intracranial neoplasms and 45%–50% of all primary malignant brain tumors. The diagnosis of GBM has traditionally been made on the basis of histologic features, including microvascular proliferation (MVP) and necrosis. Molecular information for classification of gliomas was first introduced in 2016, in the updated fourth edition of the World Health Organization (WHO) classification of CNS tumors, with introduction of isocitrate dehydrogenase (IDH)-mutant and IDH-wild-type subcategories of GBM. With robust increase in our understanding of genetics and molecular biology over the last few years, it became evident that IDH-mutant GBMs differ fundamentally from IDH-wild-type counterparts, in terms of metabolism, epigenetics, biologic behavior, aggressive infiltration, and response to therapy.1,2 A leading neuropathologist and geneticist, as a part of the Consortium to Inform Molecular and Practical Approaches to CNS Tumor Taxonomy-Not Official WHO (cIMPACT-NOW) also established that IDH wild-type diffuse astrocytic tumors without the histologic features of GBM (traditionally WHO grade II/III), could behave as high-grade (WHO grade 4) if they harbor any of the following molecular abnormalities: telomerase reverse transcriptase (TERT) promoter mutation, epidermal growth factor receptor (EGFR) amplification, or chromosomal +7/−10 copy changes. These findings by the consortium were the basis for major changes to the definition and classification scheme of GBM with removal of IDH-mutant GBM as an entity and introduction of the concept of “molecular” GBM.1⇓-3 Important advancements have been made in the field of molecular testing with chromosomal microarray, next-generation sequencing (NGS) and O6-methylguanine-DNA-methyltransferase (MGMT) methylation status evaluation becoming routine in the evaluation of GBM at major cancer centers. Detailed knowledge about these molecular markers is beyond the scope of practice for the neuroradiologist; however, a basic understanding is still needed to help integrate this information with our clinical practice This paper provides case examples of “molecular” and “histopathological” subtypes of GBM to highlight the difference between these 2 categories and to serve as a basic primer on molecular biology of GBM. There is active ongoing research in radiology focusing on identification of imaging signature of these molecular changes.
Case 1 (“Molecular” GBM)
A 39-year-old right-handed man presented with an episode of tonic-clonic seizure. The patient had begun to notice intermittent episodes of numbness on the right side of his face over the preceding month. Neurologic examination revealed normal motor strength in all extremities with normal speech and vision. There were no gait or other cerebellar abnormalities.
Imaging.
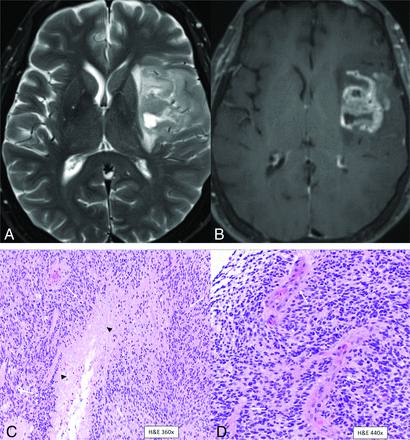
MR imaging revealed a well-circumscribed T2/FLAIR hyperintense cortical-subcortical mass centered in the frontal operculum with minimal edema, no T2/FLAIR mismatch, and no necrotic changes (Fig 1A, -B). No hemorrhagic changes or calcification was noted on the SWI (Fig 1C). The tumor showed diffuse hyperintensity on DWI with areas of low ADC values along the periphery of the mass (Fig 1D, -E). A contrast-enhanced image (Fig 1F) showed minimal patchy enhancement within the mass. Perfusion study was not performed. Primary imaging differential was a low-grade glioma (likely oligodendroglioma) and neurosurgical consult was recommended.
Left frontal lobe mass in a 39-year-old man with histologic low grade (WHO grade 2) and positive chromosomal 7 and 10 mutations supporting the molecular profile of “Glioblastoma, IDH-wild-type (CNS WHO grade 4).” Multiple axial MR images reveal a well circumscribed T2/FLAIR hyperintense cortical-subcortical mass (A and B, arrows) involving the frontal operculum with minimal edema and no internal necrotic changes. No hemorrhagic changes are noted on the SWI (C). DWI (D) and ADC map (E) reveal area of restricted diffusion with low ADC vales along the periphery of the mass. Contrast-enhanced image (F) shows minimal patchy enhancement within the mass.
Operative Report.
The patient underwent awake left frontotemporal craniotomy by using a frameless stereotaxis technique with speech and motor mapping, for resection of the mass. Presumed preoperative diagnosis was low-grade glioma. Intraoperative motor mapping posterolateral to the tumor evoked paresthesias in the right face and lip. The involved presumed motor cortex and sensory cortex was covered with cotton patties. No definite areas for speech were encountered on testing the exposed cortex for fluency, naming, and repetition. Series of corticotomy were made preserving the vascular arcades and gross total resection of the solid tumor was achieved. Tumor had well-defined borders anteriorly and less distinct margins posteriorly. Intraoperative frozen section evaluation confirmed glioma, favored to be low-grade.
Pathology and Genetics.
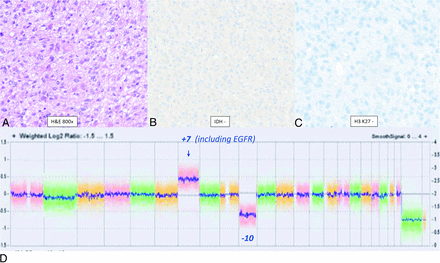
The resection specimen demonstrated a hypercellular glioma composed of round to ovoid tumor astrocytic cells with scant eosinophilic cytoplasm and occasional vague perinuclear clearing (Fig 2A). The infiltrating glioma cells were negative for IDH1-R132H and for H3K27M on immunohistochemistry (Fig 2B, -C). The cells showed retained expression of ATRX with no significant overexpression. Chromosomal microarray analysis performed by using molecular inversion probes on a whole genome array revealed combined gain of chromosome 7 (including EGFR amplification) and loss of chromosome 10 (Fig 2D, arrow). There were no features of IDH-mutant gliomas such as 1p/19q codeletion and no reportable alterations were identified in the targeted regions of the IDH1 and IDH2 genes. Neuro-oncology expanded gene panel by using NGS was also performed to evaluate for microsatellite instability status, somatic mutations, and rearrangements involving 160 genes associated with tumors of the CNS, with identification of EGFR fusion (exon 24: exon 10). Despite the absence of MVP and tumor necrosis, findings were diagnostic of glioblastoma (CNS WHO grade 4) given the molecular profile, in the IDH-wild-type setting, according to the 2021 WHO classification of CNS tumors (CNS5) classification. Tumor tissue was negative for MGMT promoter methylation. The final diagnosis based on molecular features was GBM, IDH-wild-type (CNS WHO grade 4).
Left frontal lobe mass in a 39-year-old man with histologic low grade (WHO grade 2) and positive chromosomal 7 and 10 mutations (+7/−10) supporting the molecular profile of “Glioblastoma, IDH-wild-type (CNS WHO grade 4).” H&E slide reveals moderate cytoplasm with eosinophilic processes and lack of palisading necrosis or MVP. The infiltrating glioma cells are negative for IDH1-R132H (B) and for H3 K27M(C) on immunohistochemistry. The cells showed retained expression of ATRX with no significant overexpression. Chromosomal microarray analysis performed by using molecular inversion probes on a whole genome array reveals combined gain of chromosome 7 (including EGFR amplification) and loss of chromosome 10 (D, arrow). There are no features of IDH-mutant gliomas such as 1p/19q codeletion and no reportable alterations are identified in the targeted regions of the IDH1 and IDH2 genes. Neuro-oncology expanded gene panel by using next-generation sequencing was also performed to evaluate for microsatellite instability status, somatic mutations, and rearrangements (fusions and abnormal transcript variants) involving 160 genes associated with tumors of the CNS, which confirmed the change seen on chromosomal microarray. Despite the absence of MVP and tumor necrosis, this molecular profile is now considered to be diagnostic of glioblastoma (CNS WHO grade 4) in the IDH-wild-type setting, according to the 2021 WHO Classification of CNS Tumors. Tumor tissue was negative for MGMT promoter methylation.
Case 2 (“Histopathological” GBM)
A 65-year-old woman was referred to our institution after a brain mass was discovered on head CT, done at an outside facility for strokelike symptoms. She had 2 episodes of right-sided numbness and expressive aphasia in the past months, which lasted approximately 15 minutes each. Neurologic examination was unremarkable with normal motor strength and absence of any visual or cerebellar symptoms.
Imaging.
MR imaging revealed a large heterogeneous T2-signal cortical-subcortical mass centered in the left frontal operculum with areas of necrosis evident on T2-weighted image (Fig 3A). Multiple foci of petechial hemorrhage were seen within the mass on SWI with no areas of restricted diffusion/low ADC. Contrast-enhanced images revealed thick irregular peripheral enhancement with nonenhancing areas of central necrosis (Fig 3B). There was moderate surrounding perilesional edema with mild mass effect and midline shift. The primary radiographic differential was high-grade glioma, likely GBM. Metastasis was felt to be unlikely in the absence of any underlying malignancy and given the solitary nature of the mass.
Left frontal lobe mass in a 65-year-old woman with characteristic MR imaging and histopathological features of glioblastoma, IDH-wild-type (CNS WHO grade 4). Axial T2-weighted (A) and contrast-enhanced (B) images reveal a heterogenous T2-signal cortical-subcortical mass centered in the left frontal operculum with areas of necrosis and heterogeneous peripheral enhancement. H&E slide reveals the classic “pseudopalisading” necrosis characterized by a “garlandlike” arrangement of hypercellular tumor nuclei (C, arrows) lining up around a central clear zone of tumor necrosis (C, arrowhead). Microvascular proliferation is noted with delicate capillary-like microvessels resembling classic angiogenesis, multi-layered stratification of the endothelial cells and occlusion of the vessel lumen (D, arrows). The infiltrating glioma cells were negative for IDH1-R132H and for H3 K27M on immunohistochemistry. Chromosomal microarray analysis was performed by the by using molecular inversion probes on a whole genome array revealing genomic alterations include loss of 2q22.1 (including LRP1B), 2 copy gain of chromosome 7, loss of 9p21.3 (including CDKN2A and CDKN2B), and loss of 10p15.3p11.1. Tumor tissue was negative for MGMT promoter methylation. Final integrated diagnosis was “Glioblastoma, IDH-wild-type (CNS WHO grade 4).”
Operative Report.
Stereotactic needle biopsy was done for histopathological confirmation and to plan for optimal treatment including further surgery and/or radiation with chemotherapy. The navigation device was registered by using facial surface mapping based on preoperative MR imaging. Left frontal approach was used, keeping medial to the midpupillary line to avoid biopsying near the Sylvian fissure. Intraoperative frozen section evaluation confirmed glioma, likely high-grade.
Pathology and Genetics.
Histopathological examination by using H&E and immunohistochemical stains revealed a high-grade infiltrating glioma with marked nuclear atypia, high cellularity, brisk mitotic activity, MVP, and palisading necrosis, histologically corresponding to a high-grade infiltrating glioma (Fig 3C, -D). The tumor was negative for IDH1-R132H, had retained ATRX expression, and was positive with glial fibrillary acidic protein (GFAP). Ki67 showed a high level of labeling and p53 was positive only in a subset of cells. Genomic alterations on chromosomal microarray included loss of 2 copy gain of chromosome 7, focal homozygous loss of 9p21.3 (including CDKN2A and CDKN2B), and loss of 10p15.3p11.1. Tumor tissue was negative for MGMT promoter methylation. Final diagnosis based on histopathological and molecular features was GBM, IDH-wild-type (CNS WHO grade 4).
DISCUSSION
The WHO CNS tumor classification represents the primary source of updates on diagnostic classes, grades, and criteria. The characterization and grading of CNS tumors has conventionally been on the basis of histopathology. It has now been established that such a classification scheme is not an accurate predictor of a tumor’s biologic behavior. Molecular and genetic markers provide more information with lower likelihood of undersampling, even in small biopsy specimens. Molecular information provides higher diagnostic accuracy, offering more precise prognosis and optimized treatment options, forming important elements of personalized medicine, and eventually resulting in better outcome.2,3 Molecular information in CNS tumor classification was for the first time formally incorporated in the definition of these tumors in 2016. The 2016 WHO classification represented an update of the fourth edition of WHO classification (2007) rather than being the formal fifth edition. The 2016 CNS WHO divided GBM into 1) GBM, IDH-wild-type (approximately 90% of cases), corresponding most frequently with the clinically defined “primary” or de novo GBM (>55 years of age); 2) GBM, IDH-mutant (approximately 10% of cases), which corresponds closely to so called “secondary” GBM with a history of prior lower-grade diffuse glioma (younger age-group), and 3) GBM, NOS where IDH evaluation could not be performed. GBM was previously classified as “glioblastoma multiforme” where “multiforme” referred to the highly variable histopathological features. Given the emphasis on molecular (IDH) markers in the revised fourth edition (2016) of WHO classification, the term “multiforme” was removed and these tumors were classified simply as “glioblastoma.”4 Since 2016, ongoing discoveries in molecular pathology have led to better understanding and further refinement of the classification. Given the importance of the molecular markers, an international group of leading neuropathologists (all directly involved in establishing the WHO 2016 classification) formed the cIMPACT-NOW to provide updates before the release of the next WHO classification scheme. One of the major updates by the consortium (cIMPACT-NOW) was identification of molecular clues for recognizing histologically lower-grade diffuse IDH-wild-type astrocytic gliomas, behaving as GBM.
The histologic findings of necrosis and/or MVP (Fig 3C, -D) are the traditional pathognomic hallmarks for diagnosis of GBM. However, even if these pathologic changes are absent, most histologically lower-grade (WHO 2/3) diffuse IDH-wild-type gliomas in adult patients behave as WHO grade 4 lesions.1,3 Despite the absence of necrosis and MVP, these tumors have an aggressive clinical course, with overall patient survival times not significantly different from the “histopathological” glioblastomas. This led to the proposal of new molecular criteria that could be used to upgrade these low-grade tumors. These were briefly labeled as “diffuse astrocytic glioma, IDH-wild-type, with molecular features of GBM, WHO grade IV,” by cIMPACT, however, are now simply called “glioblastoma.”3,5 The new CNS5 WHO classification defines glioblastoma as “diffuse, astrocytic glioma that is IDH-wild-type and H3-wild-type and has one or more of the following histologic or genetic features: MVP, necrosis, TERT promoter mutation, EGFR gene amplification, +7/−10 chromosome copy-number changes (CNS WHO grade 4).”2 By definition, IDH-wild-type GBM lacks immunostaining for IDH1 and is negative for H3.3 G34 mutations and H3 K27 alterations. Nuclear immunostaining for ATRX is retained in most tumors.1,3 GBM has also traditionally been divided into primary and secondary; the former arising de novo (90%) in older patients and the latter developing from a pre-existing lower-grade tumor (10%) in a younger population.7 With the development of molecular criteria, these concepts have been outdated along with removal of the term “glioblastoma multiforme” in favor of glioblastoma.8 Diffuse astrocytoma, IDH-wild-type, CNS WHO grade2/3 without molecular or histopathological features of glioblastoma is rare and is no longer regarded as a tumor type in the new classification. If there are no histopathological or molecular features of GBM in an IDH-wild-type glioma, testing for other mutations should be done (Fig 4). This includes H3.3 G34 mutation seen in diffuse hemispheric glioma, H3.3 G34-mutant (WHO grade 4) and H3 K27 alterations seen in diffuse midline glioma (WHO grade 4). H3 G34–mutant diffuse hemispheric gliomas can be excluded by immunohistochemistry for H3.3 p.G35R (G34R) or H3.3 p.G35V (G34V) mutation or by H3-3A (H3F3A) sequencing.1⇓-3,5
Chart illustration of categorization of WHO (5th) 2021 Classification of IDH-wild type diffuse astrocytic glioma. The chart highlights the role of molecular markers (TERT, EGFR, +7/−10) for diagnosis of GBM in the absence of classic histopathological findings (necrosis and MVP). The diagram also highlights the role of H3.3 G34 mutation and H3 K27 alteration in IDH-wild-type gliomas and segregation of these mutated entities from GBM.
Detailed knowledge about these mutations is beyond the scope of practice for the neuroradiologist. However, it is important to have a basic understanding of the major molecular markers, as they are frequently discussed in multidisciplinary tumor boards and correlated with radiographic patterns. MVP is one of the major histologic features of glioblastoma featuring rapid new blood vessel growth (Fig 3D) and forms the basis for the anti-vascular endothelial growth factor therapies such as bevacizumab (Avastin). The other major histologic feature of glioblastoma is necrosis. There are several postulated mechanisms by which necrosis develops in glioblastoma secondary to a microenvironment that is acidic, low in oxygen and glucose. As a result, the proliferating tumor cells migrate away from the hostile microenvironment resulting in the palisading appearance on histology (Fig 3C). IDH-wild-type gliomas have a higher incidence of intratumoral microthrombi compared to IDH-mutated gliomas due to the expression of tissue factor and podoplanin. This contributes to necrosis and an increased risk of venous thromboembolism.9 As per the WHO guidelines, absence of immunoreactivity for IDH1 p.R132H is sufficient to diagnose IDH-wild-type GBM in a patient aged ≥55 years, who has a histologically classic GBM not located in midline structures and no history of a pre-existing lower-grade glioma. This practical method is feasible because the probability of a noncanonical IDH mutation is <1% in GBM from patients aged ≥55 years. In patients aged <55 years, or in patients with a history of lower-grade glioma, negative IDH1 p.R132H immunostaining should be followed by DNA sequencing for less common IDH1 or IDH2 mutations. When no IDH mutations are detected by sequencing, tumors are classified as GBM, IDH-wild-type.3,10,11 However, genetic analysis in the form of chromosomal microarray or NGS is routinely performed at major centers to better understand tumor biology and for targeted therapies. Molecular and cytogenetic testing in neuro-oncology includes NGS, chromosomal microarray, and MGMT promoter methylation status evaluation. Chromosomal microarray provides high-resolution assessment of copy number variations across the genome, is easier to use with less complicated and less labor-intensive sample preparation than NGS. NGS evaluates mutations and rearrangements in 160 genes, including most abnormalities described by the WHO with rapid drop in cost over the last few years. MGMT promoter methylation status may be evaluated by multiple methods, and the testing platform with most prospective clinical trial validation is methylation-specific polymerase chain reaction (evaluating downstream CpG dinucleotides sites).12
TERT promoter mutation is seen in approximately 50%–60% of adults with diffuse glioma, primarily identified in oligodendroglioma (>95%) and glioblastoma. TERT promoter mutation has been associated with more aggressive behavior in GBM, however shows better outcome in low-grade gliomas. TERT results in maintenance of telomere length and plays a major role in tumorigenesis by allowing cancer cells to repress cellular senescence. Overall, approximately 60% of patients with GBM have modification of EGFR, most frequently in the form of amplification. EGFR encodes a tyrosine kinase receptor with amplification resulting in overactivation of multiple pro-oncogenic signaling pathways.13,14 Tumors with frequent EGFR alterations with co-occurrence of PTEN and TERT promoter mutations are more frequently multifocal.7 Whole chromosome 7 gain (trisomy 7) and whole chromosome 10 loss (monosomy 10) are the most frequent chromosomal copy-number aberrations in GBM, usually occur as a combination, and are commonly associated with EGFR amplification.15 Although not a part of the WHO essential diagnostic criteria for glioblastoma, MGMT promoter methylation status is listed as a desirable criterion. It is one of the leading determinants of prognosis and predictor of response to alkylating agents such as temozolomide (TMZ). MGMT promoter methylation is an independent prognostic marker for overall survival in GBM with more than 90% of longer-term surviving patients with GBM having MGMT promoter methylation/hypermethylation. Patients with MGMT promoter methylation/hypermethylation benefit more from adjuvant TMZ with more frequent pseudoprogression on imaging. Lack of MGMT promoter methylation is associated with resistance to TMZ.16,17 Few human neoplasms are as morphologically heterogeneous as GBM, and this variability can be seen in both the molecular and histopathological categories of GBM. Histologic varieties of GBM such as giant cell glioblastoma, epithelioid glioblastoma, and gliosarcoma, were added as “variants/subtypes” in the 2016 WHO classification. Although these morphologic varieties are still acknowledged in the new CNS5 edition, these are no longer defined as “subtypes” of GBM.1,4 For example, epithelioid GBM is a histologic subtype of glioblastoma defined by a mostly sharply demarcated aggregate of large epithelioid cells, which is prognostically a more favorable tumor of children and young adults and shows prominent genetic overlap (BRAF p.V600E mutation) with pleomorphic xanthoastrocytoma (Online Supplemental Data).
The most common imaging appearance of GBM is a heterogeneous mass centered in the supratentorial white matter with central necrosis, areas of hemorrhage with thick irregular enhancement, extensive peritumoral edema, and mass effect. Histologic features of MVP (neoangiogenesis) and necrosis are the major drivers for the these radiographics findings, and in the absence of these pathologic changes, molecular GBM may show only mild or patchy contrast enhancement or may lack central necrosis (Online Supplemental Data).1⇓–3 In one of the recent studies by Guo et al, it has been shown that molecular GBM was diagnosed at a younger age, had higher incidence of epilepsy, and was less likely to have contrast enhancement and intratumoral necrosis on MR imaging. Importantly, despite the lower likelihood compared to their histopathological counterparts, enhancement and necrosis were observed in most molecular GBM patients (78.8% and 63.6%, respectively) (Online Supplemental Data). Also, a small proportion of patients with histologic GBM showed absence of contrast enhancement and necrosis (<5% and 15%, respectively) (Online Supplemental Data).1 Early studies have shown that MR imaging features can predict molecular features of glioblastoma with absent histopathological changes by using a wide range of models by using distinct imaging lexicons and radiomic features. Park et al18 used the standardized feature set of Visually AcceSAble Rembrandt Images (VASARI) and radiomics extracted from the ROIs on T2WI and postcontrast T1 images to predict molecular subtype of GBMs. Studies have particularly focused on the imaging signature of EGFR mutations given the high potential for gene-based targeted therapies. Studies have shown that EGFR-mutated tumors have higher rCBV, lower ADC, higher fractional anisotropy, lower T2/FLAIR signal, and more variable spatial pattern; however, recognizing the limitation that individual assessment of these features is not sufficient to identify the mutation.19 Ivanidze et al20 in a comparison of TERT promoter mutated and nonmuated GBM showed that significantly fewer TERT promoter mutated GBM had nonenhancing tumor that crossed midline. No other imaging features were significantly different between the TERT promoter mutated and nonmutated group. It is important to note that none of these studies have conclusive imaging findings to differentiate low-grade (WHO 2/3) glioma with or without molecular features of GBM. There is, however, robust ongoing research by using radiogenomics, primarily texture analysis, for prediction of these molecular changes, as targeted therapies take center stage in treatment of gliomas. Radiomics is a rapidly growing field that utilizes computational algorithms to extract macro- and micro-scale subtle subvisual cues embedded in routine MR imaging that enables distinction of molecularly distinct GBM phenotypes. Genomewide RNA expression analysis has become a routine tool in biomedical research and RNA sequencing data can be used to noninvasively predict immune subtypes of adult diffuse gliomas by using a biologically interpretable radiomic signature from MR imaging. Newer, more powerful analytical methods such as gene set enrichment analysis (GSEA) are now being used to interpret gene expression data.21 In recent years, several notable studies have contributed to this field utilizing advanced image analysis techniques, such as morphologic and texture analysis methods, for increasing diagnostic accuracy. Choi et al22 used RNA sequencing with GSEA to calculate the radiomics features from the contrast-enhancing tumor and peri-tumoral edema regions and classify GBM into 3 different subtypes, with distinctly different prognosis and survival outcomes. Deep convolutional neural networks are used to extract features of the MR images, which are then fed to latest state-of-the-art classifier models like the “support vector machine” with highly accurate results. Tumor microenvironment is a key target area for the next-generation treatment strategies for gliomas. Radiomics has the potential to be used as a noninvasive approach to predict tumoral immunologic microenvironment and predict response to immunotherapies.22,23
Case Summary
Major changes were made to the definition of glioblastoma in the new 2021 (CNS5) classification of CNS tumors, with absence of IDH mutation (IDH-wild-type) being an essential criterion for diagnosis
Histologically lower-grade (WHO grade 2/3) diffuse gliomas behave clinically similar to GBM (grade 4) if they are IDH-wild-type
When MVP and necrosis are lacking, molecular criteria that support the diagnosis of glioblastoma include TERT promoter mutation, EGFR gene amplification, +7/−10 chromosome copy-number changes
Early studies have shown that MR imaging features with machine learning techniques can establish accurate models to identify the molecular subtypes of GBM
Terms such as “primary” and “secondary” GBM and “glioblastoma multiforme” are outdated and should be avoided.
Footnotes
Disclosure forms provided by the authors are available with the full text and PDF of this article at www.ajnr.org.
References
- Received January 24, 2024.
- Accepted after revision February 29, 2024.
- © 2024 by American Journal of Neuroradiology