Abstract
BACKGROUND AND PURPOSE: The choroid plexus contains specialized ependymal cells responsible for CSF production. Recent studies have demonstrated volumetric and perfusion changes in the choroid plexus with age and neurodegenerative disorders, however, volumetric changes in the choroid plexus in low pressure states is not known. The purpose of this study is to evaluate volumetric differences in choroid plexus size in patients with spontaneous intracranial hypotension (SIH) resultant from spinal CSF leaks compared with healthy controls.
MATERIALS AND METHODS: This was a retrospective, institutional review board–approved study. Patients with MRI evidence of SIH and a spinal CSF leak diagnosed on myelography and subsequently confirmed at surgery were included in this study. All patients included in this study including age-matched healthy controls had a brain MRI performed on a either a 1.5 or 3T scanner with acquisition of 3D T1 postcontrast (eg, BRAVO, MPRAGE, etc). In all patients, the trigonum ventriculi volume, in the atria of the lateral ventricles, was contoured by using Visage-7 segmentation tools on the volumetric postcontrast T1 sequence. A basic 2-tailed t test was used to compare choroid plexus volumes between the 2 groups.
RESULTS: Thirty-four patients were included with 17 patients with SIH with spinal CSF leak and 17 healthy control patients who were age- and sex-matched. The mean age of patients was 45 years, standard deviation 14 years. The mean volume of the choroid plexus for patients with SIH with spinal CSF leak was 1.2 cm3 (standard deviation = 0.26) compared with 0.63 cm3 (standard deviation = 0.31) in the control group (P < .0001).
CONCLUSIONS: Results of this study demonstrate a higher choroid plexus volume in patients with SIH with spinal CSF leak compared with age- and sex-matched healthy controls. This likely reflects compensatory mechanisms to counteract intracranial hypotension by increasing CSF production as well as increased vascularity of the choroid plexus through expansion of the intracranial blood pool.
ABBREVIATIONS:
- ICHD-3
- International Classification of Headache Disorders, 3rd edition
- SIH
- spontaneous intracranial hypotension
The choroid plexus is a specialized structure within the ventricular system tasked with modulating CSF production and the expression of various proteins, neurotrophic factors, and neuroinflammatory cytokines.1⇓-3 The choroid plexus contains fenestrated capillaries and thus lacks a blood-brain barrier. Age-related changes within the choroid plexus affecting volumetric size and function have been previously described noting a reduction in CSF production and the accumulation of calcification with increasing age.4⇓-6 Recent interest in changes to function and size of the choroid plexus have grown due to its possible relationship to the glymphatic system with potential implications in neurodegenerative processes and other neuroinflammatory/immune-mediated disorders.7⇓⇓⇓⇓⇓⇓⇓-15
Before this recent resurgence of interest in the choroid plexus, earlier studies have focused on the role of the choroid plexus in hydrocephalus associated with hyperplasia.16,17 However, to date, the role of the choroid plexus in modulating states of relatively low or decreasing amounts of CSF has not been investigated. Numerous brain imaging markers associated with intracranial hypotension have been previously described including distention of the dural venous sinuses, engorgement of the pituitary gland, features of brain sagging, diffuse pachymeningeal enhancement, and presence of extra-axial fluid collections.18⇓-20 Many of these features have demonstrated a high validity in the prediction of a spinal CSF leak being detected on myelography, termed the Bern score.21 However, a description in size and volumetric differences in the choroid plexus in the setting of intracranial hypotension due to conditions such as a spinal CSF leak have yet to be investigated.
The purpose of this study was to evaluate differences in choroid plexus volume in patients with spontaneous intracranial hypotension (SIH) secondary to spinal CSF leaks compared with age-matched healthy controls.
MATERIALS AND METHODS
This was a retrospective, institutional review board–approved study performed at a single institution with a robust spinal CSF leak service with greater than 80 patient visits per year for evaluation of spinal CSF leak. Inclusion criteria for study patients included: 1) adult patients; 2) those with imaging features of SIH on MRI; and 3) those who had a spinal CSF leak diagnosed on CT myelography that was subsequently confirmed upon surgical repair. Inclusion criteria for control patients included: 1) adult patients; 2) those who had a brain MRI with contrast; 3) those who had no history of migraines or chronic headaches; and 4) those who also had no clinical features, imaging features, or symptoms of SIH. All patients with SIH with a confirmed spinal CSF leak met inclusion criteria for SIH based on the International Classification of Headache Disorders, 3rd edition (ICHD-3) criteria.22 All healthy control patients were those undergoing a brain MRI with contrast as a known healthy control participant in neuroimaging research or those undergoing a brain MRI with contrast for evaluation of a suspected pituitary microadenoma. Exclusion criteria for both the study patients and control patients were those with significant artifacts on MRI precluding a diagnostic assessment, those with incomplete medical records, any history of neurosurgery, hydrocephalus or any history of a ventricular shunt placement, malignancy, craniofacial radiation, history of vasculopathy/vasculitis, intracranial vascular malformation (including dural arteriovenous fistula, arteriovenous malformations, Sturge Weber syndrome, etc), history of intracranial infection, multiple sclerosis, or suspicion for a neurodegenerative process.
All brain MRI examinations included in this study were performed with gadolinium contrast and included an isotropic 3D T1 sequence (such as BRAVO, MPRAGE, etc). All MRI studies were performed over a 3-year time period from 2020–2023. Examinations were performed across multiple MRI vendor platforms and included 1.5T and 3T magnet strengths. Before inclusion of this study, all MRI examinations were reviewed for quality by a single neuroradiologist (author K.B.).
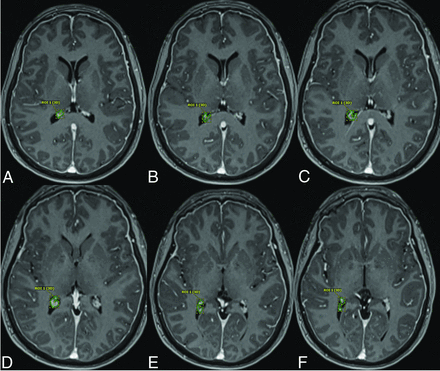
3D volumetric contouring was manually performed in Visage 7 by using the structure segmentation toolkit (Visage version 7.1.18; Visage Imaging). Manual segmentation of the choroid plexus on the left and right side in each patient was performed independently and obtained from 2D axial images (Fig 1). The choroid plexus at the trigonum ventriculi was targeted for segmentation. The trigonum ventriculi is the triangular cavity of the lateral ventricle at the junction of the lateral ventricular body and the occipital and temporal horns. This methodology for choroid plexus segmentation was adapted from the work of Senay et al.23 The choroid plexus volumes were independently calculated for the left and right side in all patients and were counted as 2 independent and discrete data points (ie, 2 choroid plexus volumes per patient). Choroid plexus cysts and xanthogranulomas if present were excluded from the choroid plexus contours.
Forty-three-year old man with SIH who was found to have a type 3 spinal CSF leak. Axial T1 postcontrast MPRAGE sequences demonstrating manual segmentation of the right choroid plexus as described in the Materials and Methods section.
Basic demographic data including patient age, sex, type of CSF leak found in patients with SIH, and reason for MRI evaluation were obtained. The Bern score, a probabilistic scoring system for likelihood of spinal CSF leak based on brain MRI findings, was calculated for all patients with SIH with spinal CSF leaks as well as for control patients (for the purposes of this study).21,24 Control patients and patients with SIH with spinal CSF leaks were age- and sex-matched.
Statistical analysis by using a 2-tailed t test was used to compare choroid plexus volumes between patients with SIH with spinal CSF leak and age- and sex-matched healthy control patients.
RESULTS
A total of 34 patients (for a total of 68 recorded choroid plexus volumes) were included in this study including 17 patients with SIH with confirmed spinal CSF leak and 17 healthy control patients. The mean age of patients with SIH with confirmed CSF leak was 45.1 years (standard deviation = 14.5 years). The mean age of the healthy control patients was 45.3 years (standard deviation = 14.3 years) with no significant difference found between the age of study patients and control patients (P = .86). There were 8 female and 11 male patients comprising both the patients with spinal CSF leak and the healthy control patients for sex matching of the cohort.
Of the 17 patients with a confirmed spinal CSF leak, 10 had a type 1 spinal CSF leak (dural tear from an osseous spicule), 2 had a type 2 spinal CSF leak (root sleeve diverticular defect), and 5 had a type 3 spinal CSF leak (CSF-venous fistula). Bern score calculations for each of the patients with SIH related to a spinal CSF leak ranged from 5–9 (mean = 7.8, standard deviation = 1.4), all in keeping with a high probability for a spinal CSF leak. While the healthy control patients had no clinical or imaging features to suggest SIH related to any cause, a Bern score was calculated for all healthy control patients. All healthy control patients had a Bern score of 0.
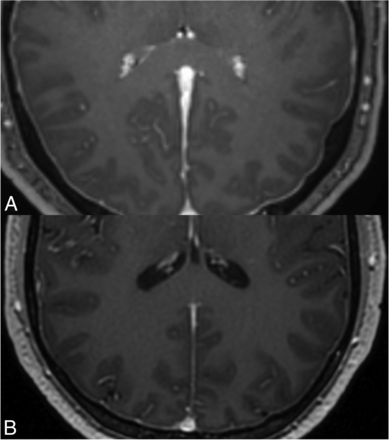
All patients with CSF leak had at least 1 MRI brain imaging feature of intracranial hypotension, while none of the controls had imaging features of intracranial hypotension. The mean volume of the choroid plexus for study patients with SIH from a spinal CSF leak was 1.2 cm3 (standard deviation = 0.26, interquartile range = 0.31) compared with 0.63 cm3 (standard deviation = 0.31, interquartile range = 0.42) (P < .0001) in healthy control patients (Fig 2). A summary of this demographic, clinical, and imaging data are displayed in the Table.
Axial T1 postcontrast MPRAGE sequences through the level of the atria of the lateral ventricles. A, (top) shows the caliber of the choroid plexus in a 34-year-old man with a type 1 CSF leak. Additionally shown in this image is diffuse thickening of the pachymeninges, a frequent finding in patients with intracranial hypotension. B, (bottom) shows the caliber of the choroid plexus at the same level in a 35-year-old male healthy control.
Basic demographic, clinical, and imaging features for patients with SIH with spinal CSF leaks compared with healthy control patients
DISCUSSION
The results of this study demonstrate a statistically significant difference in choroid plexus volumes in patients with spinal CSF leaks and brain MRI manifestations of intracranial hypotension compared with age-matched healthy control patients. Larger choroid plexus volumes were observed in patients with SIH related to spinal CSF leaks compared with healthy control patients. This finding may be resultant from compensatory mechanisms of the choroid plexus to increase CSF production to replace progressive CSF loss related to the spinal CSF leak.1-2,25,26 Furthermore, the larger choroid plexus volumes could reflect a response to CSF leakage through aquaporin-4-channel up-regulation or increased glymphatic system clearance.27 Interestingly, a prior study demonstrated larger choroid plexus volumes in multiple sclerosis compared with both neuromyelitis optica and healthy controls, which may further support aquaporin-4-channel up-regulation as a physiologic response.27 Additionally, both hyperplasia and vascular engorgement from expansion of the intracranial blood pool may contribute to higher choroid plexus volumes in patients with SIH with spinal CSF leaks. There may potentially be other unknown mechanisms at play in this setting.
There are several limitations to this study in that the sample size is relatively small. Future direction includes conducting this analysis on a large cohort of patients to investigate the generalizability of these results to a large population. This small sample size is in line with prior studies examining volumetric changes in the choroid plexus. Additionally, in this study only volumetric size was evaluated and more functional markers of the choroid plexus such as perfusion metrics were not investigated. Furthermore, additional potential confounders such as medication history (eg, carbonic anhydrase inhibitor, among others), body mass index, and blood pressure were not taken into account in this study, which may have a potential impact on study results.
CONCLUSIONS
Preliminary results from this pilot study demonstrate volumetric differences with larger choroid plexus volumes in patients with spinal CSF leaks compared with healthy controls likely reflecting underlying compensatory mechanisms. The results of this study may be potentially useful in the preprocedural evaluation of patients with suspected spinal CSF leak as an additional diagnostic predictor. Furthermore, choroid plexus volume measurements may potentially be used to evaluate the posttreatment efficacy of these patients, though larger and more robust studies need to be performed to validate the results herein.
Footnotes
Disclosure forms provided by the authors are available with the full text and PDF of this article at www.ajnr.org.
References
- 1.
- 2.
- 3.
- 4.
- 5.
- 6.
- 7.
- 8.
- 9.
- 10.
- 11.
- 12.
- 13.
- 14.
- 15.
- 16.
- 17.
- 18.
- 19.
- 20.
- 21.
- 22.
- 23.
- 24.
- 25.
- 26.
- 27.
- Received February 12, 2024.
- Accepted after revision March 8, 2024.
- © 2024 by American Journal of Neuroradiology